Bonding
advertisement

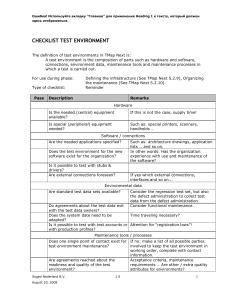
Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Bonding and Structure 726814716 Page 1 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. CHEMICAL BONDING Stable atoms The noble gases exist as single atoms that are very stable. All electrons are paired and the bonding shells are full. helium neon argon Bonding Compounds form when atoms are joined together by forces called chemical bonds. When bonds form, unpaired electrons often pair up to form a noble gas electron structure. This is often referred to as the ‘octet rule’. the bonding shells are full the electron structure is very stable. Types of bonding Bonds are classified into two main types: ionic and covalent. As a general rule, Ionic bonding occurs between a metal and a non-metal. Covalent bonding occurs between a non-metal and a non-metal. Questions Predict the type of bonding in the following compounds. (a) sodium chloride …………………. (d) silver bromide (b) zinc oxide …………………. (e) nitrogen bromide …………………. (c) hydrogen chloride …………………. (f) sulphur dioxide 726814716 Page 2 of 45 …………………. …………………. © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. IONIC BONDS Ionic bonds are present in a compound of a metal and a non-metal. Electrons are transferred from a metal atom to a non-metal atom forming ions. The metal ion is positive The non-metal ion is negative An ionic bond is the electrical attraction between oppositely charged ions. Sodium chloride, NaCl Na atom Cl atom Na+ ion Cl ion noble gas neon argon The ions that are formed often have stable noble gas electron structures with full outer electron shells. Magnesium chloride, MgCl2 Mg atom 726814716 Cl atoms Mg2+ ion Cl ions noble gas neon argon Page 3 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Problems (a) Draw ‘dot-and-cross’ diagrams to show how atoms can form the four ionic compounds below. (b) Write down the noble gas that would have the same electron structure as each ion that you draw. (i) magnesium oxide, ….. atom (ii) ….. ion noble gas ……… ……….. ….. atom ….. ion ….. ion noble gas ……… ……….. ….. atom ….. ion ….. ion noble gas ……… ……….. aluminium oxide. ….. atom 726814716 ….. ion sodium oxide, ….. atom (iii) ….. atom Page 4 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. IONS AND THE PERIODIC TABLE The charge on an ion can easily be predicted from its position in the Periodic Table. Complete the table below. Group 1 2 3 4 5 6 7 8 number of outer shell electrons 1 2 3 4 5 6 7 8 element Li Be B C N O F Ne 2,1 2,2 2,3 2,4 2,5 2,6 2,7 2,8 ion element Li+ F 2 2,8 Na Mg Al Si P S Cl Ar 2,8,1 2,8,2 2,8,3 2,8,4 2,8,5 2,8,6 2,8,7 2,8,8 ion Multiple charges Sometimes elements can form ions with different charges. The charge is shown by a Roman numeral. iron(II) for Fe2+ and iron(III) for Fe3+; copper(I) for Cu+ and copper(II) for Cu2+. Predicting ionic formulae calcium chloride: equalise charge formula 726814716 ion charge ion charge Ca2+ 2+ Cl 1– Ca2+ 2+ 2 Cl 2– CaCl2 Page 5 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Predicting formulae 1. Predict the formula of the ionic compounds below. (a) lithium chloride …………………. (e) aluminium fluoride …………………. (b) sodium oxide …………………. (f) aluminium sulphide…………………. (c) beryllium oxide …………………. (g) aluminium phosphide………………. (d) lithium nitride …………………. (h) magnesium nitride…………………. 2. Predict the formula of the ionic compounds below. (a) copper(II) oxide …………………. (e) cobalt(II) oxide (b) copper(I) oxide …………………. (f) chromium(III) oxide……………….. (c) iron(II) fluoride …………………. (g) manganese(VI) oxide………………. (d) iron(III) chloride …………………. (h) vanadium(V) nitride…………………. 726814716 Page 6 of 45 ……..…………. © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Molecular ions common molecular ions ion 1+ 1– 2– OH– ammonium NH4+ hydroxide 3– carbonate CO32– phosphate PO43– nitrate NO3– sulphate SO42– nitrite NO2– sulphite SO32– Ionic compounds from molecular ions sodium carbonate equalise charge ion charge ion charge Na+ 1+ CO32– 2– 2 Na+ 2+ 2 CO32– 2– formula calcium nitrate equalise charge Na2CO3 ion charge ion charge Ca2+ 2+ NO3– 1– Ca2+ 2+ 2 NO3– 2– formula Ca(NO3)2 Predicting more formulae Predict the formula of the ionic compounds below. …………………. (a) lithium nitrate …………………. (e) iron(III) sulphite (b) sodium carbonate …………………. (f) chromium(III) nitrite ………………. (c) aluminium sulphate…………………. (g) ammonium phosphate ……………. (d) calcium hydroxide …………………. vanadium(V) sulphate………………. 726814716 (h) Page 7 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. COVALENT BONDS A covalent bond is formed between atoms of non-metals with a similar attraction for electrons. A covalent bond is a shared pair of electrons. A covalent bond forms when two atoms attract the same pair of electrons. H2 molecule Cl2 molecule Molecules with different atoms (a) Draw ‘dot-and-cross’ diagrams to show a molecule of the following compounds of hydrogen. (b) Write down the formula of each compound. (c) What is the common name given to each compound? hydrogen chloride hydrogen oxide formula: …………. formula: …………. common name: ………………….. common name: ………………….. hydrogen nitride hydrogen carbide formula: …………. formula: …………. common name: ………………….. common name: ………………….. 726814716 Page 8 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. 726814716 Page 9 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Multiple bonds Double and triple bonds are possible. O2 N2 Drawing ‘dot-and-cross’ diagrams 1 2 Draw dot-and-cross diagrams of the following molecules of (i); (ii); (iii); (iv), F2 HF SiF4 SCl2 Draw dot-and-cross diagrams of the following molecules, each containing at least one multiple covalent bond. 726814716 CO2 C2H4 HCN H2CO Page 10 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. DATIVE COVALENT BONDS A dative covalent bond forms when the shared pair of electrons comes from just one of the atoms. The formation of the ammonium ion, NH4+. NH3 H+ NH4+ More covalent questions 1. Draw dot-and-cross diagrams to show the formation of a dative covalent bond in the oxonium ion, H3O+. 2. (a) Draw a dot-and-cross diagram for a molecule of boron trifluoride. (b) How many electrons surround the boron atom? ............................................................................................................................... (c) In what way, is the electron structure different to those met previously? ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (d) Why do you think this structure is still stable? ............................................................................................................................... ............................................................................................................................... 726814716 Page 11 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. 3. When covalent bonds form, unpaired electrons often pair up to form a noble gas electron structure. This is often referred to as the ‘octet rule’. Many molecules, however, form electron structures that do not form an octet. Draw ‘dot-and-cross’ diagrams for the following molecules which do not obey the octet rule. 726814716 AlCl3 PCl5 SF6 SO3 SO2 SO3 Page 12 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. SHAPES OF MOLECULES the shape of a molecule depends upon the number of electron pairs surrounding the central atom. The shapes of simple molecules can be explained using electron-pair repulsion. The pairs of electrons that surround an atom repel one another. When this happens the electron pairs become as far apart as possible. The electron-pairs naturally repel into the following shapes around a central atom. molecule number of electron pairs around central atom dot-and-cross diagram shape bond angle BCl2 BF3 CH4 SF6 726814716 Page 13 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Molecules with lone pairs A lone pair of electrons repels more than a bonded pair. This is because a lone pair is closer to the atom: In general: lone-pair/lone-pair repulsion > bonded-pair/lone-pair repulsion > bonded-pair/lone-pair repulsion bonded-pair/bonded-pair repulsion Lone pairs will distort the shape of a molecule and reduce the bond angle: molecule number of dot and cross diagram lone pairs shape bond angle CH4 NH3 H2O 726814716 Page 14 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Some shape questions For each of the following molecules, draw a dot-and-cross diagram, and predict the shape and bond-angles. molecule number of dot and cross diagram lone pairs shape bond angle BeF2 AlCl3 SiH4 H2S PH3 726814716 Page 15 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Double bonds A double bond is treated in the same way as a bonded pair. Each double bond and pair of electrons should be treated as a bonding ‘area’. molecule number of bonding areas ‘dot and cross’ diagram shape bond angle CO2 Now try these… molecule number of bonding areas ‘dot and cross’ diagram shape bond angle CS2 C2H4 SO3 SO2 726814716 Page 16 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. IONIC OR COVALENT? An ionic bond with 100% ionic character would require the complete transfer of an electron from a metal atom to a non-metal atom. In practice, this never completely happens! Between the extremes of ionic and covalent bonding, there is a whole range of intermediate bonds, which have both ionic and covalent contributions. Ionic bonds with covalent character Many ionic compounds have a degree of covalency resulting from incomplete transfer of electrons. This occurs in ionic compounds with a small + ion (cation) with a high charge density and a large – ion (anion) with a low charge density. charge density = Ошибка! 1 + 2 + 3 + C h a rg e /s iz e ra tio in c re a s e s The electric field around a small cation distorts the electron shells around a large anion. This effect is called polarisation. As a result, electrons are attracted towards the cation, giving a degree of covalency (electron sharing). In Al2O3, polarisation takes place between Al3+ and O2– ions. 2 - 3 + A l O 3 + S m a ll A l io nish ig h lyc h a rg e d . Oio nisd is to rte da n dp o la ris e d re s u ltin ginp a rtia lc o v a le n tc h a ra c te r. 726814716 Page 17 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. ELECTRONEGATIVITY The nuclei of the atoms in a molecule attract the electrons pair in a covalent bond. It is this attraction which is responsible for the covalent bond. I n a h y d r o g e n m o l e c u l e ,t h e n u c l e u s o f e a c h h y d r o g e n a t o m i s a t t r a c t e d t o w a r d s t h e e l e c t r o n p a i r o ft h e c o v a l e n t b o n d . -+ + H H Electronegativity is a measure of the attraction of an atom in a molecule for the pair of electrons in a covalent bond. The most electronegative atoms attract bonding electrons most strongly. Elements with small atoms have the most electronegative atoms. Highly reactive non-metallic elements (such as O, F and Cl) have the most electronegative atoms. Reactive metals (such as Na and K) have the least electronegative atoms. How is electronegativity measured? electronegativity increases Li 1.0 Na 0.9 K 0.8 Be 1.5 B 2.0 C 2.5 N 3.0 O 3.5 F 4.0 Cl 3.0 Br 2.8 Fluorine is the most electronegative element with an electronegativity of 4.0; Fluorine has small atoms which attract the pair of electrons in a covalent bond more strongly than larger atoms. The greater the difference between electronegativities, the greater the ionic character of the bond. The greater the similarity in electronegativities, the greater the covalent character of the bond 726814716 Page 18 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. POLAR AND NON-POLAR MOLECULES Non-polar bonds A covalent bond is non-polar when the bonded electrons are shared equally between both atoms the bonded atoms are the same the bonded atoms have similar electronegativities. A covalent bond must be non-polar if the bonded atoms are the same. 726814716 HH Cl Cl H2 molecule Cl2 molecule Page 19 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Polar bonds A covalent bond is polar when the electrons in the bond are shared unequally making a polar bond; the bonded atoms are different, each has a different electronegativity. In hydrogen chloride the H–Cl bond is mainly covalent with one pair of electrons shared between the two atoms. However, the chlorine atom is more electronegative than the hydrogen atom. The chlorine atom attracts the bonded pair of electrons more than the hydrogen atom making the covalent bond polar. The electrons in the bond are shared unequally making a polar bond. + H Cl HCl molecule The HCl molecule is polarised with a small positive charge + on the H atom and a small negative charge – on the Cl atom. The hydrogen chloride molecule is polar with a permanent dipole. Symmetrical and unsymmetrical molecules In symmetrical molecules, dipoles cancel and there are no permanent dipoles. dipoles cancel CHCl3 - polar CCl4 - non-polar In CCl4 each C–Cl bond is polar but the dipoles act in different directions the overall effect is for the dipoles to cancel each other 726814716 Page 20 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. CCl4 is a non-polar molecule. Some polar questions 1. 2. Draw simple diagrams for each of the following molecules. Decide whether each molecule is polar. If so, use the periodic table to predict the dipole present and shows these clearly as + and on your diagrams. (i) Br2 H2O O2 HBr NH3 CF4 Predict the shape of a molecule of BF3 and of PF3. BF3 (ii) PF3 Explain why BF3 is non-polar whereas PF3 is polar. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 3. (i) Predict the shape of a molecule of H2O and of CO2. H2O (ii) CO2 Explain why H2O is polar whereas CO2 is non-polar. ............................................................................................................................... ............................................................................................................................... 726814716 Page 21 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. ............................................................................................................................... ............................................................................................................................... INTERMOLECULAR FORCES Strength of bonds and forces Ionic, covalent and metallic bonds are of comparable strength. Intermolecular forces are much weaker: type of bond covalent bond hydrogen bond van der Waals’ forces bond enthalpy/ kJ mol1 200-500 5-40 2 VAN DER WAALS' FORCES Van der Waals’ forces (induced dipole-dipole interactions) exist between all molecules whether polar or non-polar. Without these forces, non-polar molecules could never form a liquid or a solid. Van der Waals’ forces are weak intermolecular interactions caused by attractions between very small dipoles in molecules. movement of electrons produces an oscillating dipole - + + - dipole oscillates and continually changes with time - - + - + oscillating dipole induces - + - + - + a dipole in a neighbouring molecule which is induced onto further molecules induced dipoles attract one another + What affects the strength of van der Waals’ forces? Van der Waals' forces result from interactions of electrons between molecules. The greater the number of electrons in each molecule the larger the oscillating and induced dipoles 726814716 Page 22 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. the greater the attractive forces between molecules the greater the van der Waals' forces. 726814716 Page 23 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Van der Waals’ forces and electrons Molecules with more electrons will generate larger oscillating and induced dipoles. These produce larger attractive forces between molecules. The table below shows the boiling points of the hydrogen compounds of group 4. compound CH4 SiH4 GeH4 SnH4 boiling point /K 112 161 178 221 number of electrons 1. Complete the table above with the number of electrons in each compound. 2. Explain why the boiling point increases down this group. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 3. Compare these boiling points with those for the hydrogen compounds of Group 6 (page 4). Explain the similarities and differences between the two sets of data. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 726814716 Page 24 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. ............................................................................................................................... 726814716 Page 25 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. PERMANENT DIPOLE-DIPOLE INTERACTIONS Permanent dipole-dipole interaction are weak intermolecular forces. They are simply attractions between weak dipole charges on different molecules. Permanent dipole-dipole interactions are ‘non-directional’. Intermolecular forces between HCl molecules H Cl weak dipole-dipole interactions between HCl molecules H H Cl H Cl Cl H Cl Between HCl molecules, there will be both weak intermolecular forces: van der Waals’ forces and permanent dipole-dipole interactions. permanent dipole-dipole interactions > van der Waals’ forces. Now do this….. Draw a diagram to show the dipole-dipole interactions that exist between molecules of nitrogen monoxide, NO. 726814716 Page 26 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. HYDROGEN BONDS A hydrogen bond is a special type of permanent dipole-dipole interaction found between molecules containing the following groups: H O H N H F - Hydrogen bonds act between: a lone pair HO:, HN: or HF: and a hydrogen atom HO, HN or HF on a different molecule. Hydrogen bonding occurs between molecules such as H2O. Hydrogen bonding is important in organic compounds containing –OH or –NH bonds: e.g. alcohols, carboxylic acids, amines, amino acids. When water change state, the covalent bonds between the H and O atoms in an H2O molecule are strong and do not break - the much weaker intermolecular forces break 726814716 Page 27 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Some hydrogen bonding questions 1. Put a tick below the following molecules that have hydrogen bonding: molecule hydrogen bonding? 2. H2O H2S CH4 HOCH3 NH3 NO2 H2NCH3 Each molecule that you draw should show relevant lone pairs and dipoles. Draw diagrams showing hydrogen bonding between (a) 2 molecules of ammonia; (b) 2 molecules of hydrogen fluoride; (c) 2 molecules of ethanol, C2H5OH; (d) 1 molecule of water and 1 molecule of ethanol. 726814716 Page 28 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. SPECIAL PROPERTIES OF WATER A hydrogen bond has only about one-tenth the strength of a covalent bond. However, hydrogen bonding is strong enough to have significant effects on physical properties, resulting in some unexpected properties for water. The solid (ice) is less dense than the liquid (water) Particles in solids are usually packed closer together than in liquids. Hydrogen bonds hold water molecules apart in an open lattice structure. ice is less dense than water. The diagram below shows how the open lattice of ice collapses on melting. hydrogen bonds break MELTING OF ICE ice lattice collapses: molecules move closer together tetrahedral open lattice in ice H2O has a relatively high melting point and boiling point There are relatively strong hydrogen bonds between H2O molecules. The hydrogen bonds are extra forces, over and above van der Waals’ forces. These extra forces result in higher melting and boiling points than would be expected from just van der Waals’ forces. When the ice lattice breaks, hydrogen bonds are broken. Other properties The extra intermolecular bonding from hydrogen bonds also explains the relatively high surface tension and viscosity in water. 726814716 Page 29 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. How do hydrogen bonds affect the properties of water? Hydrogen bonds increase the attraction between molecules. This will result in an increase in the boiling point of a compound. The table below shows the boiling points of the hydrogen compounds of group 6. H2O boiling point /K 373 H2S 213 H2Se 231 H2Te 270 compound relative molecular mass 1. Complete the table above with the relative molecular mass of each compound. 2. Plot a graph of boiling point against relative molecular mass. Join each point with a line. 3. The boiling point of water is higher than expected owing to hydrogen bonding. Use your graph to estimate what the boiling point of water would be if there were no hydrogen bonding. Show this clearly on your graph. 4. Refer to Chapter 3 of the Foundation Chemistry textbook. Water has several peculiar properties that can be explained by hydrogen bonds. The unexpected high boiling and melting point is just one of these. Explain how hydrogen bonding is responsible for three more unusual properties of water. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 726814716 Page 30 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. BONDING, STRUCTURE AND PROPERTIES The properties of a substance depend upon its bonding and structure. Ionic and covalent bonds are of comparable strength - Intermolecular forces are far weaker. Ionic bonds break when a giant ionic lattice melts or boils Simple molecular structure – weak forces break - low melting point Covalent bonds break when a giant molecular lattice melts or boils Giant structure – strong forces break - high melting point GIANT IONIC LATTICES Ionic compounds form giant ionic lattices with each ion surrounded by ions of the opposite charge. A giant ionic lattice is held together by strong electrostatic attraction between positive and negative ions. Each ion is surrounded by oppositely-charged ions, forming a giant ionic lattice. Part of the sodium chloride lattice, Na+ Cl - Each Na+ ion surrounds 6 Cl ions Each Cl ion surrounds 6 Na+ ions Although it is convenient to look at ionic bonding between two ions only, each ion is able to attract oppositely charged ions in all directions. This results in a giant ionic lattice structure with hundreds of thousands of ions (depending upon the size of the crystal). This arrangement is characteristic of all ionic compounds. 726814716 Page 31 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Properties of Giant ionic lattices High melting point and boiling point Ionic compounds are solids at room temperature. The attraction is strong between + and ions. High temperatures are needed to break the strong electrostatic forces holding the ions rigidly in the solid lattice. ionic compounds have high melting and boiling points. Electrical conductivity In the solid lattice, the ions are in a fixed position and there are no mobile charge carriers. an ionic compound is a non-conductor of electricity in the solid state. When melted or dissolved in water, the solid lattice breaks down, the ions are now free to move as mobile charge carriers. an ionic compound is a conductor of electricity in liquid and aqueous states. Solubility The ionic lattice dissolves in polar solvents (e.g. water). The polar water molecules break down the lattice and surround each ion in solution as shown below for sodium chloride. 726814716 Page 32 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. PROPERTIES OF COVALENT COMPOUNDS Elements and compounds with covalent bonds have either of two structures: a simple molecular structure, a giant molecular structure. SIMPLE MOLECULAR STRUCTURES Simple molecular structures have small molecules, such as Ne, H2, O2, N2. A simple molecular structure is held together by weak forces between molecules The atoms within each molecule are bonded strongly together by covalent bonds Properties of simple molecular structures Low melting point and boiling point Low temperatures provide sufficient energy to break the intermolecular forces. simple molecular structures have low melting and boiling points. Strong covalent bonds hold together each I2 molecule Weak van der Waals' forces between I2 molecules When the simple molecular structure of I2 is broken, only the weak van der Waals’ forces between the I2 molecules break; the covalent bonds, I–I, are strong and do not break. Electrical conductivity There are no free charged particles. simple molecular structures are non-conductors of electricity. Solubility Van der Waals' forces form between a simple molecular structure and a non-polar solvent, such as hexane. These interactions weaken the structure. 726814716 Page 33 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. simple molecular structures are soluble in non-polar solvents (e.g. hexane). 726814716 Page 34 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. GIANT MOLECULAR STRUCTURES Diamond, graphite and SiO2 are common examples of giant molecular lattices. This type of structure is known by a variety of names: a giant molecular lattice, a giant covalent lattice, a giant atomic lattice and a macromolecular lattice. A giant molecular structure is held together by strong covalent bonds between atoms. Properties of giant molecular structures High melting point and boiling point High temperatures are needed to break the strong covalent bonds in the lattice. giant molecular structures have high melting and boiling points. Electrical conductivity Except for graphite (see below), there are no free charged particles. giant molecular structures are non-conductors of electricity. Solubility The strong covalent bonds in the lattice are too strong to be broken by either polar or non-polar solvents. giant molecular structures are insoluble in polar and non-polar solvents. 726814716 Page 35 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. PROPERTIES OF DIAMOND AND GRAPHITE diamond property graphite tetrahedral hexagonal layers symmetrical structure held together by strong covalent bonds throughout lattice strong layer structure but with weak bonds between the layers poor conductivity good conductivity There are no delocalised electrons All outer shell electrons are used for covalent bonds. hard Delocalised electrons between layers. Electrons are free to move parallel to the layers when a voltage is applied. soft Tetrahedral shape enables external forces to be spread throughout the lattice. Strong bonding within each layer weak forces between layers easily allow layers to slide. structure electrical conductivity hardness 726814716 Page 36 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. BONDING AND STRUCTURE IN METALS Metallic bonding A metallic bond holds atoms together in a solid metal or alloy. In solid metals, the atoms are ionised. The positive ions occupy fixed positions in a lattice; The outer shell electrons are delocalised – they are spread throughout the metallic structure and are able to move freely throughout the lattice. A metallic bond is the electrostatic attraction between the positive metal ions and delocalised electrons. In the metallic lattice, each metal atom exists as + ions by releasing its outer shell electrons to the ‘sea of electrons’. + - + - + - - + - + - + - + - - + - + - + The ‘sea of electrons’ Delocalised and localised electrons In a metallic bond, the delocalised electrons in metals are spread throughout the metal structure; the delocalised electrons are able to move throughout the structure; it is impossible to assign any electron to a particular positive ion. In a covalent bond, the localised pair of electrons is always positioned between the two atoms involved in the bond; the electron charge is concentrated between the bonded atoms. 726814716 Page 37 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. PROPERTIES OF GIANT METALLIC LATTICES All metals form giant metallic lattices in the solid state A giant metallic lattice is held together by strong electrostatic attractions between positive ions and negative electrons. A metallic bond acts between the positive metal ions and delocalised electrons. High melting point and boiling point Generally high temperatures are needed to separate the ions from their rigid positions within the lattice. most metal have high melting and boiling points. Good thermal and electrical conductivity The existence of mobile, delocalised electrons allows metals to conduct heat and electricity well, even is the solid state. - + + - + + - + - + + - + - + + + + + + + drift of delocalised electrons across potential difference The electrons are free to flow between positive ions. The positive ions do not move. When a metal conducts electricity, only the electrons move. Solubility Metals are insoluble. Solvents such as water are unable to form strong enough forces with the ions and electrons to pull the lattice apart. 726814716 Page 38 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. COMPARISON OF STRUCTURE, BONDING AND PROPERTIES The different types of bonds and forces Covalent bonds act between atoms. Ionic bonds act between ions. Metallic bonds act between positive ions and electrons. Hydrogen bonds act between polar molecules. Dipole-dipole interactions act between polar molecules. Van der Waals' forces act between induced dipoles of molecules. structure m pt/ b pt reason electrical conductivity reason solubility reason giant ionic simple molecular giant molecular hydrogen bonded giant metallic 726814716 Page 39 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. Properties questions 1. Magnesium oxide forms a similar ionic lattice to that of sodium chloride. (a) Draw a clear diagram of the magnesium oxide lattice. (b) Predict, with reasons, the following properties of magnesium oxide: (i) melting and boiling points ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (ii) electrical conductivity ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (iii) solubility. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (c) Suggest, with reasons, why magnesium oxide has a higher melting point than sodium chloride, ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 726814716 Page 40 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. ............................................................................................................................... 726814716 Page 41 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. 2. (a) Describe what is meant by hydrogen bonding, ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (b) Describe how hydrogen bonding influences the properties of water. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... 726814716 Page 42 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. ............................................................................................................................... 726814716 Page 43 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. 3. The data below gives some properties of four substances, A, B, C and D. compound solubility in water solubility in hexane A B C D good poor poor poor insoluble good poor poor (a) electrical conductivity aqueous solid solution poor good poor poor poor good - boiling point /K 1738 456 2503 3160 Explain how these data suggest different structures for the four substances shown. ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... ............................................................................................................................... (b) Suggest an identity for each substance. A ................................................................................................................. B ................................................................................................................. C ................................................................................................................. 726814716 Page 44 of 45 © Rob Ritchie, 08/04/20 Ошибка! Используйте вкладку "Главная" для применения Title к тексту, который должен здесь отображаться. D ................................................................................................................. 726814716 Page 45 of 45 © Rob Ritchie, 08/04/20