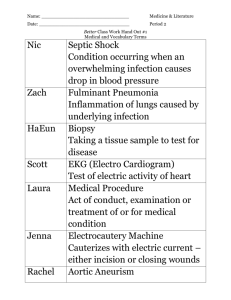
GMY302 10202
advertisement

PLQ Incorporated Page 1 of 2 Reason Narrative Written: Death: SAE: Discontinuation due to adverse event: Discontinuation due to laboratory abnormality: Protocol: CANCER PROTOCOL Subject identifier: 10202 Subject demographics: 79-year-old white female Treatment group: CANCERX + dexamethasone Date of first dose of study drug: CANCERX: 16 Apr 2002 Dexamethasone: 19 Apr 2002 Date of last dose of study drug (study day): CANCERX: 27 Jan 2003 (Day 287) Dexamethasone: 24 Jan 2003 (Day 284) Preferred term for adverse event: Spinal compression fracture, hydropneumothorax, urinary tract infection, sepsis methicillin-resistant Staphylococcus aureus, injection site infection Narrative: Subject 10202 was diagnosed with multiple myeloma in Apr 1998. The subject previously received treatment with cytoxan, vincristine, carmustine, cyclophosphamide, melphalan, prednisone, vertebroplasty, and radiotherapy. The subject’s pertinent medical history included thoracic back pain, recurrent urinary tract infections, cholelithiasis, depression, constipation, anemia, and allergies to penicillin and sulfa. On 15 May 2002 (Day 30; Cycle 2, day 2 of therapy), the subject was admitted to the hospital for management of severe back pain. Earlier that day, she had fallen and immediately began having back pain that radiated down her left side. An x-ray showed an L4 compression fracture, which was confirmed with a computed tomography scan. The subject had some decreased sensation in the left foot but no other sensory deficits. Her lungs had coarse bronchial sounds. The subject was treated with rofecoxib, morphine, and IV fluids. The lumbar compression fracture was considered resolved with residual effects on 18 May 2002 (Day 33), and the subject was discharged. Relevant concomitant medications for the event of spinal compression fracture included pantoprazole, pamidronate, calcitonin, gabapentin, senna, famciclovir, epoetin alfa, ferrous sulfate, paroxetine, and hydrocodone with acetaminophen. On 19 Jun 2002 (Day 65; Cycle 3 of therapy), the subject presented to the emergency department with a temperature of 38.9oC and complaints of headache and dizziness. A chest x-ray showed a large, left pleural effusion with an air-fluid level suggestive of hydropneumothorax. The subject had not experienced any recent trauma that could have caused a hydropneumothorax. On 20 Jun 2002, a chest x-ray showed 20% pneumothorax with little change from the previous day. A computed tomography scan of the head, performed as an assessment of the subject’s headaches, showed extensive lytic destruction in the calvarium with opacification of the left frontal air cell and destruction of the anterior bony wall of the left frontal sinus. The subject underwent ultrasound-guided, left-sided thoracentesis for removal of approximately 400 mL of cloudy yellow fluid, which contained polymorphonuclear leukocytes (4+) and red blood cells (3+) but was negative for pathological organisms. The subject received treatment with ketorolac, cefotaxime, vancomycin, acetylcysteine, and hydrocodone. The subject was discharged on 23 Jun 2002, and she was considered recovered from the hydropneumothorax on 01 Jul 2002 (Day 77). Study medication was temporarily interrupted from 20 Jun to 08 Jul 2002 as a result of the event. Last saved: 12 Jun 2003 PLQ Incorporated Page 2 of 2 Relevant concomitant medications for the event of hydropneumothorax included oxycodone, calcitonin, gabapentin, paroxetine, senna, famciclovir, ferrous sulfate, epoetin alfa, hydrocodone with acetaminophen, pantoprazole, and pamidronate. On 20 Nov 2002 (Day 219; 2 days after completing Cycle 9 therapy), the subject had poor oral intake, nausea, and vomiting. On 23 Nov 2002 (Day 222; 5 days after completing Cycle 9 therapy), she was admitted to the hospital due to intractable back pain, a temperature of 38.6 oC, and leukocytosis. Physical examination revealed mild tenderness to palpation of the abdomen and thoracic spine as well as poor skin turgor. Her WBC count was elevated (13,600/L) with 85% neutrophils. Her slightly cloudy urine had WBCs present. Urine cultures grew Escherichia coli. The subject was diagnosed with urinary tract infection, which was considered the cause of her back pain. She was treated with acetaminophen, morphine, levofloxacin, and vancomycin. The urinary tract infection was considered resolved on 28 Nov 2002 (Day 227). The subject’s hospitalization was prolonged due to sepsis with methicillin-resistant Staphylococcus aureus, which was diagnosed on 26 Nov 2002 from blood cultures. The sepsis was treated successfully with vancomycin. The methicillin-resistant Staphylococcus aureus infection was considered resolved on 09 Dec 2002 (Day 238), and the subject was discharged on that day. Relevant concomitant medications for the events of urinary tract infection and methicillin-resistant Staphylococcus aureus sepsis included pamidronate, oxycodone, paroxetine, senna, famciclovir, ferrous sulfate, gabapentin, pantoprazole, and hydrocodone with acetaminophen. On 18 Dec 2002 (Day 247, Cycle 10), the subject was admitted to the hospital after developing a fever of 38.4oC, weakness, and clammy skin. At admission, the subject was pale and had a blood oxygen saturation of 88% on room air. She was treated with 4 L/min of oxygen via nasal cannula. After blood cultures from her central venous catheter were positive for Staphylococcus, the central venous catheter was replaced, and the subject began treatment with cefotaxime, gentamicin, and vancomycin. The injection site infection resolved and the subject was discharged on 23 Dec 2002 (Day 252). Infusion of CANCERX was interrupted temporarily on 20 Dec 2002 while a new central venous catheter was inserted. Relevant concomitant medications for the event of injection site infection included oxycodone, gabapentin, paroxetine, senna, famciclovir, hydrocodone with acetaminophen, ferrous sulfate, triamcinolone, acetaminophen, polyethylene glycol, prednisone, vancomycin, zoledronic acid, and pantoprazole. In the opinion of the investigator, the spinal compression fracture was moderate (Grade 2), unlikely related to CANCERX, and unlikely related to dexamethasone. The hydropneumothorax was considered moderate (Grade 2), unlikely related to CANCERX, and unrelated to dexamethasone. In the opinion of the investigator, the urinary tract infection, sepsis methicillin-resistant Staphylococcus aureus, and injection site infection were severe (Grade 3), unlikely related to CANCERX, and unlikely related to dexamethasone. Last saved: 12 Jun 2003