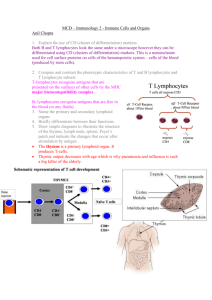
BOX 7-1 Genetic Blocks in Lymphocyte Maturation
advertisement

BOX 10-1 Transgenic Mouse Models for the Analysis of Tolerance and Autoimmunity The experimental analysis of self-tolerance is confounded by two important technical problems. First, it is not possible to identify self-reactive lymphocytes by functional assays because these cells are normally deleted or functionally inactive (anergic). Second, in normal animals or humans, it has been difficult or impossible to define the nature, tissue distribution, and levels of expression of self antigens, particularly MHC-associated peptide antigens for T cells. For these reasons, much of our early understanding of tolerance was based on administering tolerogenic forms of foreign antigens to animals and studying subsequent immune responses to immunogenic forms of the same antigens. Conclusions about self-tolerance were largely extrapolations from these studies with foreign antigens. Transgenic technology has provided a valuable tool for studying self-tolerance in mice. Rearranged antigen receptor genes can be expressed as transgenes in T or B lymphocytes (see Appendix III). Because these antigen receptor genes inhibit recombination at other, endogenous, antigen receptor gene loci (the phenomenon of allelic exclusion), a large fraction of the T or B lymphocytes in these mice express the introduced, transgene-encoded antigen receptor. Therefore, lymphocytes with a known specificity may be detected and followed quantitatively for the life of a mouse. The second application of transgenic technology is to express known proteins in different tissues. These transgene-encoded antigens are present throughout the development of the animal, and therefore they are effectively self antigens for the mouse. Transgenic approaches may be used to study self-tolerance in many ways. The maturation and functional responsiveness of self antigen-specific lymphocytes may be followed in mice by expressing an antigen receptor specific for a normally expressed self antigen. This was first done by expressing a class I MHC-restricted TCR specific for the male antigen H-Y in CD8+ T cells. The T cells fail to mature in male mice because they are negatively selected in the thymus when the immature cells encounter H-Y peptides (see Chapter 7, Fig. 7-21). The same principle has been exploited to study B cell tolerance to a self class I MHC molecule by expressing a membrane Ig specific for one class I allele in B cells. In mice containing that class I allele, the B cells are eliminated in the bone marrow or they change their specificity. A variation of this approach is to express transgenic antigen receptors specific for self antigens that are targets of autoimmune diseases. Examples include mice expressing a TCR specific for a protein in pancreatic islet β cells (a target for autoreactive T cells in type I diabetes), a TCR specific for myelin basic protein (which is a central nervous system autoantigen), and Ig specific for self DNA (involved in the autoimmune disease lupus). These transgenic mice are useful for defining not only the mechanisms of self-tolerance but also the pathogenesis of reactions that serve as models of human autoimmune diseases. Transgenic models may be made even more amenable to analysis by coexpressing both the antigen receptors of T or B lymphocytes and the antigen that is recognized by these receptors. Two examples we mention in the text are T cells specific for a viral glycoprotein expressed in islet β cells and B cells specific for hen egg lysozyme expressed in different tissues. By changing the promoters used to drive transgene expression, it is possible to vary the site of expression of the antigen. The use of inducible promoters allows investigators to turn the expression of the antigen on and off during the life of the mouse. It is also possible to express the same antigen in different forms (secreted, membrane bound, and cytoplasmic) and thus to analyze tolerance to different types of self antigens. Genes encoding particular immunoregulatory molecules, such as costimulators and cytokines, may be coexpressed with antigens, thus modeling the consequences of local alterations in the tissues where particular self antigens are present. In addition, by breeding antigen receptor transgenics with appropriate knockout mice, investigators have generated mice in which lymphocytes of known specificities lack genes encoding lymphocyte regulatory molecules, such as CTLA-4 and Fas ligand. This results in selective defects in lymphocyte regulation and provides models for studying the effects of such changes on defined lymphocyte populations as well as the pathogenesis of disorders associated with mutations in these regulatory genes. Experimental systems using transgenic and knockout mice have allowed investigators to analyze the types of antigens that induce central and peripheral tolerance in T and B cells, the mechanisms of these pathways of tolerance, and the genetic control of self-tolerance. Experimental protocols have been developed to compare the consequences of self antigen recognition by immature or mature lymphocytes. For instance, as described in the text, by mating one mouse expressing a transgenic antigen receptor with another mouse expressing the antigen, in the offspring, the immature lymphocytes are exposed to the antigen throughout development. Alternatively, mature lymphocytes expressing the antigen receptor may be transferred into mice expressing the antigen as a self protein, and the consequences of this encounter may be analyzed. Despite the value of transgenic technology, several important caveats should be mentioned. Expression of a single antigen receptor markedly limits the normal lymphocyte repertoire. Transgene-encoded protein antigens are often expressed at higher concentrations than are normal self proteins. Transgeneencoded immunoregulatory molecules not only are expressed at high levels but also are expressed constitutively and constantly, which is rarely the case with normal immunoregulatory molecules. Therefore, many of the normal controls on lymphocyte activation and regulation may be lost in these transgenic mice. BOX 10-2 Apoptosis in Lymphocytes There are many situations in biology when cells normally die and are eliminated by phagocytosis without eliciting harmful inflammatory reactions. For instance, the process of embryogenesis involves modeling of tissues and organs by balanced cell division and cell death, and physiologic reductions in circulating hormone levels lead to death of hormone-dependent cells. In these situations, cell death occurs by a process called apoptosis, which is characterized by DNA cleavage, nuclear condensation and fragmentation, plasma membrane blebbing, changes in membrane lipid distribution, and detachment of cells from the extracellular matrix. Apoptotic cells are rapidly phagocytosed because they express membrane molecules that are recognized by a variety of receptors on phagocytes. This type of physiologic cell death contrasts with necrosis, in which plasma membrane integrity breaks down and cellular contents are enzymatically degraded and released, resulting in pathologic inflammation. In the immune system, apoptosis is important for eliminating unwanted and potentially dangerous lymphocytes at many stages of maturation and after activation of mature cells. In the following sections, we describe the mechanisms and regulation of apoptosis, focusing on lymphocytes. The physiologic roles of apoptosis in the immune system are discussed in this and other chapters. INDUCTION OF APOPTOSIS IN LYMPHOCYTES The induction of apoptosis involves the activation of cytosolic enzymes called caspases. Caspases are cysteine proteases (i.e., proteases with cysteines in their active sites), so named because they cleave substrates at aspartic acid residues. Caspases are present in the cytoplasm of most cells in an inactive form (also called a zymogen, referring to an inactive enzyme). In its inactive state, a caspase exists as a single polypeptide chain with a prodomain and a catalytic domain. Caspases are themselves activated by cleavage after aspartic residues, and the active caspase that is produced is a dimer with two catalytic subunits. Fourteen caspases have been identified, and the number is likely to increase. Some caspases function as initiators, to start the process of apoptosis often by cleaving and thereby activating more caspases. These activated caspases function as effectors or executioners, cleaving many substrates and leading to nuclear fragmentation and the other changes of apoptosis. In lymphocytes and most other cells, caspase activation and subsequent apoptosis may be induced by two distinct pathways, one of which is associated with mitochondrial permeability changes and the other with signals from death receptors in the plasma membrane. The mechanisms of induction of these two pathways are illustrated in Figure A, and their biochemical mechanisms are in Figure B. Cell death as a result of loss of survival stimuli: the intrinsic, or mitochondrial, pathway of apoptosis. If lymphocytes are deprived of necessary survival stimuli, such as growth factors or costimulators (for T cells), the result is rapid increase in the permeability of mitochondrial membranes and release of several proteins, including cytochrome c, into the cytoplasm. Cytochrome c functions as a cofactor with a protein called apoptosis activating factor-1 (Apaf-1) to activate an enzyme, called caspase-9, that initiates the apoptotic pathway. Other proteins released from mitochondria may directly block the normal anti-apoptotic activities of Bcl family members (described later), again resulting in cell death. This pathway of apoptosis has been called passive cell death, implying that it does not require active signals resulting from the engagement of death receptors. (Note, however, that all pathways of apoptosis are actively induced by enzymes and protein degradation.) This form of apoptosis is also called programmed cell death, or death by neglect, implying that many cells are programmed to die unless protected by survival stimuli, and they will die if neglected (not provided survival stimuli). DNA damage caused by irradiation, certain chemotherapeutic drugs, and glucocorticoids may induce apoptosis of target cells by the mitochondrial pathway. In addition, self antigen recognition may trigger mitochondrial translocation of proapoptotic members of the Bcl family (such as Bim; see text), which block the protective actions of the anti-apoptotic members, again resulting in cell death by the mitochondrial pathway. Activation-induced cell death mediated by death receptors: the extrinsic, or receptorinitiated, pathway of apoptosis. The second pathway of apoptosis in lymphocytes is triggered by the binding of ligands to death-inducing membrane receptors. The best defined death receptors belong to a family of proteins with homologous cysteine-rich extracellular domains. The first members of this family to be identified were receptors for the cytokine tumor necrosis factor (TNF), and the family includes a large number of proteins, such as Fas and CD40. The cytoplasmic regions of different members of this family contain either a conserved "death domain" or a domain that binds signaling molecules and activates transcription factors. (The pathway of transcriptional activation by TNF receptors is described in Chapter 11, Box 11-1.) The two best described death domain-containing receptors are Fas (CD95) and the type I TNF receptor; we focus on Fas as the prototype because its role in lymphocyte regulation is better established. Fas was identified as a 36kD surface protein that, on cross-linking by specific antibodies, triggered apoptosis of cells that expressed it. Lymphoid cells and many other cell types express Fas. Fas ligand (FasL) is a homotrimeric membrane protein that is expressed mainly on T lymphocytes after activation by antigen and IL-2. When mature T lymphocytes are repeatedly stimulated by antigens, they coexpress Fas and FasL. FasL binds to Fas on the same or adjacent cells, clustering three or more Fas molecules. The intracellular death domains of the clustered Fas receptors bind a cytosolic death domain-containing adapter protein called FADD (for Fas-associated death domain). FADD, in turn, binds the inactive form of a caspase, caspase-8 (see Figure B). Caspase-8 undergoes autocatalytic activation and is then able to activate effector caspases and trigger apoptosis. This pathway of apoptosis is called activation-induced cell death because it is induced by lymphocyte activation (and not by the absence of survival stimuli). The type I TNF receptor probably triggers a similar pathway of cell death. Although the type I TNF receptor does not bind FADD directly, its cytoplasmic domain does bind a homologous protein, called TRADD (for TNF receptor-associated death domain), that can recruit FADD to the complex. The physiologic role of TNF receptors in regulating lymphocyte survival and in self-tolerance is not established. Note that apoptosis induced by antigen recognition, and involving the mitochondrial pathway, has also been called activationinduced cell death, because it follows activation by the antigen. This pathway of apoptosis does not involve death receptors. Passive cell death by the mitochondrial pathway and activation-induced cell death by the death receptor pathway differ in how they are induced and, as we shall see, in their regulation and principal physiologic roles (see Table). In some nonlymphoid cells, such as hepatocytes, Fas-induced signals result in increased mitochondrial permeability, and the two death pathways may act cooperatively to trigger apoptosis. Many of the proteins known to induce and regulate apoptosis were identified as the products of genes that are homologous to genes first shown to regulate apoptosis in the worm Caenorhabditis elegans. During the development of this worm, particular cells die in a precise sequence, so that the consequences of genetic manipulations on death or survival of cells can be accurately defined. Several different ced genes (for "cell death abnormal" genes) are known, and their mammalian homologues have been identified. Caspase-9 is homologous to Ced-3, Apaf-1 to Ced-4, and the anti-apoptotic protein Bcl-2 (see below) to Ced-9. Effector mechanisms of apoptosis. Once caspase-9 or caspase-8 becomes proteolytically active, it in turn cleaves and activates other downstream effector caspases, including caspase-3 and caspase-6. These enzymes act on a variety of substrates, including nucleases and proteins of the nuclear envelope, to initiate DNA fragmentation and nuclear breakdown, the hallmarks of apoptosis. (Note that not all mammalian caspases are involved in cell death. The first of these enzymes to be identified, now called caspase-1, functions to convert the precursor form of the cytokine IL-1β to its active form and was therefore originally called the IL-1-converting enzyme [ICE]. On the basis of this name, caspase-8 was originally called FADD-like ICE or FLICE.) REGULATION OF APOPTOSIS Programmed death of lymphocytes (passive cell death) is prevented by various activating stimuli, including specific antigen recognition, growth factors (such as the cytokine IL-2), and costimulation (e.g., engagement of CD28 on T cells by B7 molecules on APCs). All these stimuli function by inducing the expression of anti-apoptotic proteins of the Bcl family. The first member of this family to be identified was called Bcl-2 because it was the second oncogene found in a human B cell lymphoma. Bcl-2, and its homologue Bcl-x, inhibit apoptosis by blocking the release of proapoptotic proteins like cytochrome c from mitochondria and by inhibiting the activation of caspase-9 (see Figure B). Several other Bcl family members have been identified that form homodimers and heterodimers and can be phosphorylated in response to growth factors, thereby regulating their activities. The details of these interactions are being investigated in many laboratories. Some proteins related to Bcl proteins are proapoptotic. For instance, a protein called Bid (which contains a domain that is homologous to domains found in Bcl proteins) binds to and blocks the activity of Bcl-2. Therefore, Bid promotes apoptosis. Another related protein called Bim, which may be induced by antigen recognition, antagonizes Bcl-2 and thus promotes apoptosis. Many other proapoptotic and anti-apoptotic members of this complex family are known. Bcl proteins do not appear to block Fas/TNF receptor-induced apoptosis in most cell types. Activation-induced cell death by the Fas pathway is prevented by a protein called FLIP (for FLICE-inhibitory protein) that has a death domain but lacks a caspase domain. FLIP may bind to the adapter protein FADD or to inactive caspase-8 in the cytoplasm, but it cannot activate the caspase. Thus, it competitively inhibits the binding of caspase-8 to the Fas-associated protein complex and blocks the apoptotic signal. Naive T cells contain high levels of FLIP, and activation of the cells in the presence of IL-2 reduces the expression of FLIP. This is why the Fas pathway is inactive in naive T cells, allowing antigen-stimulated responses to develop, but becomes active after T cell stimulation, functioning to prevent responses to repeated antigen encounter. Table I01-1. Pathways of Apoptosis in Lymphocytes Features Induced by "Passive" cell death (death by neglect) Deficiency of survival stimuli (antigen, growth factors, costimulators) Activation-induced cell death Repeated lymphocyte activation Membrane None receptors involved Death receptors (e.g., Fas) Early caspases involved Caspase-8 (and caspase-10 in humans) Caspase-9 Effect of IL-2 on T Prevents apoptosis cells Enhances apoptosis Role of Bcl proteins Prevents apoptosis No effect in most cell types Principal physiologic roles Death of immature lymphocytes that fail positive selection Death of mature cells that do not encounterantigen, and after antigen is eliminated Elimination of some self-reactive mature lymphocytes that repeatedly encounter self antigens Negative selection of immature lymphocytes (largely not Fas or TNF-R mediated) Abbreviation: TNF-R, tumor necrosis factor receptor. page 230 page 231 PHYSIOLOGIC ROLES OF APOPTOSIS IN LYMPHOCYTES Programmed, or passive, cell death plays an essential role in controlling the size of the lymphocyte pool at many stages of lymphocyte maturation and activation. Immature lymphocytes that do not express functional antigen receptors or are not positively selected die by neglect (see Chapter 7). After their maturation, if naive lymphocytes do not encounter the antigen for which they are specific, the naive cells die by apoptosis. After activation by antigen, many of the progeny of the activated cells also die as the antigen is eliminated. In all these situations, the lymphocytes do not receive the survival stimuli that would protect them from programmed cell death. As expected, overexpression of Bcl-2 or Bcl-x as a transgene in T or B lymphocytes results in enhanced survival of immature lymphocytes and prolonged immune responses. Thus, this pathway of apoptosis is critical for maintaining homeostasis in the immune system. Fas-mediated activation-induced cell death appears to be most important for preventing uncontrolled activation of lymphocytes (e.g., by abundant and persistent self antigens). Great interest in the function of Fas was spurred by the demonstration that in two inbred mouse strains that develop autoimmune disease as a result of recessive single-gene mutations, the defects lie in either Fas or FasL. These were the first systemic immune diseases shown to be a result of a failure of apoptosis. A small number of humans with similar disorders have been described. We will return to a discussion of these autoimmune disorders in Chapter 18. The negative selection of immature thymocytes that encounter high concentrations of self antigens is also due to activation-induced cell death, but it does not appear to rely on either Fas or TNF receptors. The Fas pathway may prevent uncontrolled lymphocyte activation in response to persistent infections (e.g., some viral infections), but the importance of this pathway in normal homeostasis is not established. In addition to its role in the maintenance of peripheral tolerance to self antigens, Fas:FasL-mediated cell death may serve other functions. Cytolysis by CD8+ cytolytic T lymphocytes (CTLs) is in part mediated by FasL on the CTLs binding to Fas on target cells (see Chapter 13). Two tissues known to be sites of immune privilege, namely, the testes and the eyes, may constitutively express FasL. It is postulated that FasL kills leukocytes that enter the tissues, thus preventing local immune responses, which is the hallmark of immune privilege. However, these findings are controversial and may not apply to all species. The physiologic value of immune privilege in these tissues, and in the central nervous system, is not understood. Table 10-1. Factors That Determine the Immunogenicity and Tolerogenicity of Protein Antigens Factor Factors that favor stimulation of immune responses Factors that favor tolerance Amount Optimal doses that vary for different antigens High doses Persistence Short-lived (eliminated by immune response) Prolonged (repeated T cell stimulation induces apoptosis) Portal of entry; location Subcutaneous, intradermal; absence from generative organs Intravenous, oral; presence in generative organs Presence of adjuvants Antigens with adjuvants: stimulate helper T Antigens without adjuvants: cells nonimmunogenic or tolerogenic Properties of High levels of costimulators Low levels of costimulators and antigenpresenting cells cytokines Table 10-2. Self-Tolerance in T and B Lymphocytes Feature T lymphocytes B lymphocytes Principal sites of tolerance induction Thymus (cortex); periphery Bone marrow; periphery Tolerance-sensitive stage of maturation CD4+CD8+ (double-positive) thymocyte Immature (IgM+IgD-) B lymphocyte Stimuli for tolerance induction Central: high-avidity recognition of antigen in Central: high-avidity recognition of thymus multivalent antigen in bone marrow Peripheral: antigen presentation by APCs Peripheral: antigen recognition without T lacking costimulators; repeated stimulation by cell help self antigen Principal mechanisms Central tolerance: clonal deletion (apoptosis) Central tolerance: clonal deletion of tolerance (apoptosis), receptor editing Peripheral tolerance: anergy, apoptotic cell death, suppression Peripheral tolerance: block in signal transduction (anergy); failure to enter lymphoid follicles