Graded Assignment
SCI303A/304A: Chemistry | Unit 6 | Lesson 6: Mid-Unit Test
Graded Assignment
Mid-Unit Test, Part 2
Answer the following questions. You may use the periodic table in the Chemistry: Problems and Solutions book
for this test. When you have finished, submit this assignment to your teacher by the due date for full credit.
(5 points)
Score
1.
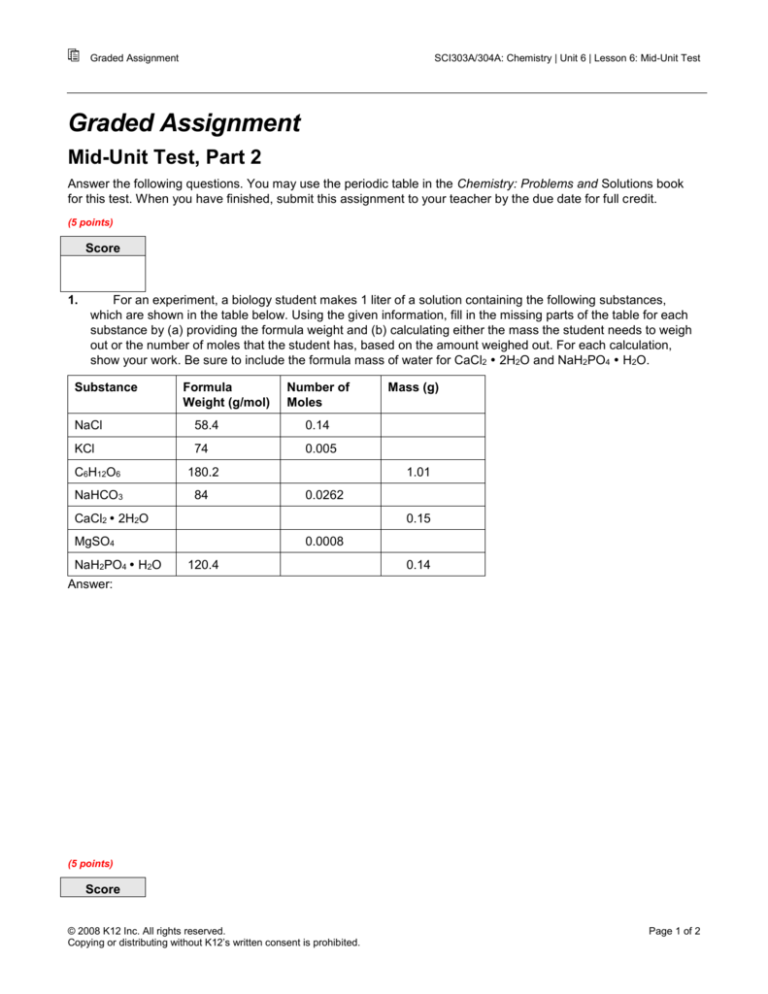
For an experiment, a biology student makes 1 liter of a solution containing the following substances,
which are shown in the table below. Using the given information, fill in the missing parts of the table for each
substance by (a) providing the formula weight and (b) calculating either the mass the student needs to weigh
out or the number of moles that the student has, based on the amount weighed out. For each calculation,
show your work. Be sure to include the formula mass of water for CaCl2 2H2O and NaH2PO4 H2O.
Substance
Formula
Weight (g/mol)
Number of
Moles
NaCl
58.4
0.14
KCl
74
0.005
C6H12O6
180.2
NaHCO3
84
1.01
0.0262
CaCl2 2H2O
0.15
MgSO4
NaH2PO4 H2O
Mass (g)
0.0008
120.4
0.14
Answer:
(5 points)
Score
© 2008 K12 Inc. All rights reserved.
Copying or distributing without K12’s written consent is prohibited.
Page 1 of 2
Graded Assignment
2.
SCI303A/304A: Chemistry | Unit 6 | Lesson 6: Mid-Unit Test
Acetaminophen (C8H9NO2) is the active ingredient in many nonprescription pain relievers. Each tablet
contains 500 mg of acetaminophen, and a typical adult dose is two tablets every eight hours.
Determine the molar mass of acetaminophen (show your work).
Calculate the number of moles of acetaminophen in a single tablet (show your work).
Calculate the number of moles of acetaminophen that an adult would have taken if she took three
doses of acetaminophen in one day (show your work).
Answer: (8 times 12) + (1 times 9)+ (1 times 14) + (2 times 16) = 151 for molar mass of acetaminophen
Molacetaminophen = 500mg divided by mass of acetaminophen = 3.311
Molacetaminophen + (500mg times 3 doses) divided by molar mass acetaminophen = 9.933
Your Score
___ of 10
© 2008 K12 Inc. All rights reserved.
Copying or distributing without K12’s written consent is prohibited.
Page 2 of 2