ALEXANDER MACKENZIE HIGH SCHOOL
advertisement

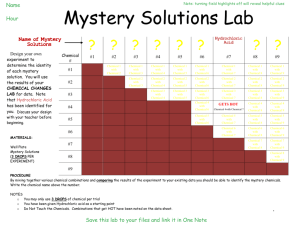
STEPHEN LEWIS SECONDARY SCHOOL SNC 1D SCIENCE CULMINATING ACTIVITY – CHEMISTRY IDENTIFY THE MYSTERY SUBSTANCE PURPOSE Students will use the terminology and knowledge gained during the Chemistry unit of study to identify a mystery substance by observing and describing various chemical and physical properties. SCENERIO Forensic science is the application of science to criminal investigations (just think C.S.I.). Forensic scientists collect, analyze and evaluate evidence from a crime scene to be used by law enforcement. Qualitative analysis (examining physical and chemical properties of a substance) is an integral part of forensic science. In this activity, you get to play the role of a C.S.I. Your team suspects that a death was caused by the ingestion of a mysterious white solid. Traces of the solid were found near the victim. The primary suspect in the case claims that a severe heart attack was the cause of death. It is your responsibility to help your team identify the mystery solid and decide whether or not it could have been the cause of death. FORMAT The lab portion of the activity will be completed in one period You will be given five known white solids and some lab equipment Your task is to test these five solids by observing and describing the various physical and chemical properties You will also be provided with the unknown sample found at the crime scene and your job is to determine the identity of the unknown solid (it’s one of the five you just finished studying) ANALYSIS Record your physical and chemical observations in the table provided and complete the C.S.I. Investigation Report Determine the identity of the mystery solid by comparing the physical and chemical properties of your unknown substance to the five known substances You will answer a set of questions that involve explaining how you arrived at your conclusions. You will also be asked questions about course content and skills in the context of the lab (just like the Mystery Gases Lab) CHEMISTRY TOPICS TO REVIEW information on a periodic table physical properties and chemical properties classification of matter pure substance vs. mixture heterogeneous mixture vs. homogeneous mixture element vs. compound classify a substance as an element or compound based on its symbol, chemical formula classify a substance as a homogeneous or heterogeneous mixture based on its appearance and composition identify metals and non-metals by their symbols identify the family a metal/non-metal belongs to (alkali, alkaline earth, halogens, noble gases) physical change (formation of a mixture, change in state, change in form) chemical change and the different types of evidences for chemical change count the number of atoms in a chemical formula atom vs. molecule recognize a molecule by its chemical formula and its picture 1 MARKING SCHEME 1. C.S.I. Investigation Report a) Completion of Table of Observations b) Correct identity of the mystery solid c) Rationale for choosing powder d) Explanation of cause of death e) 0 0 0 0 1 1 1 1 2 3 2 2 3 4 /10 2. Analysis Questions 3. Safety and Clean-up Protocol /24 0 1 2 3 4 /4 Total Mark = / 38 2 INTRODUCTION In groups of 3-4, you will be investigating the physical and chemical properties of various white solids. You will record your physical and chemical properties in the tables provided. Make sure each group member records the observations as you will be using the results to answer analysis questions. Make sure that each group member has a chance to participate in the testing. MATERIALS Eye protection Toothpick or stirring rod 5 different vials with solids Aspirin (C9H8O4) Powdered Sugar (C6H12O6) Sodium Dihydrogen Phosphate (NaH2PO4) Buffered Aspirin (many people have trouble taking aspirin because it upsets their stomach; buffered aspirin includes an extra ingredient in it known as calcium carbonate (CaCO3) to make it gentler on the stomach) Baking Soda (NaHCO3) 5 bottles that contain each of these solids mixed with water Spot plate Dilute Hydrochloric Acid, HCl(aq) Universal Indicator Dilute iron (III) (ferric) nitrate solution, Fe(NO3)3(aq) Iodine solution, I2(aq) PROCEDURE 1. Using a scoopula, add a small amount (tip of your scoopula) of each substance in its solid form to each of the wells in the SECOND ROW of the spot plate. 2. Using the dropper bottles (shake them well first) fill up the wells half way of the spot plate in the FIRST, THIRD, AND FOURTH ROW. 3. Use the diagram below to set up. Substances you will be testing Sodium Dihydrogen Buffered Baking Mystery Aspirin Sugar Phosphate Aspirin Soda Substance Universal Indicator Testers sol sol sol HCl solid solid solid Fe(NO3)3 sol sol sol sol sol sol Iodine sol sol sol solid solid sol sol sol sol sol sol solid 3 4. Record the observable physical properties of each solid and record it in Table 1. 5. Examine each of the dropper bottles containing the solids that have been mixed with water. Are these solids soluble in water? Record your observations in Table 1. 6. Add one drop of universal indicator to each of the five substances in the FIRST ROW of your spot plate. Mix the contents well using a toothpick/stirring rod. Record your observations in Table 2. 7. Add dilute hydrochloric acid to solid samples of each of the five substances in the SECOND ROW of your spot plate. Fill up the well in the spot plate to the rim. Record your observations in Table 2. 8. Add 5-6 drops of dilute iron (III) nitrate to each of the five substances in the THIRD ROW. Record your observations in Table 2. 9. Add 5-6 drops of iodine to each of the five substances in the FOURTH ROW of your spot plate. Stir. This reaction may take some time, so be sure to check it before you empty your spot plats. Record your observations in Table 2. 10. Record the letter of your mystery substance in the space provided on your data sheets. 11. Fill up your spot plate with the mystery substance. Remember to put the solid sample in the SECOND ROW and the sample mixed with water in the FIRST, THIRD, AND FOURTH row. 12. Record the physical properties of your mystery substance in the table provided. 13. Test and record the chemical changes of your mystery substance in the table provided. 14. Identify your mystery substance as either Aspirin, Powdered Sugar, Sodium Dihydrogen Phosphate, Buffered Aspirin or Baking Soda. 15. Clean your spot plate well using water and leave everything at your lab bench the same way you found it. Show your teacher to get your full marks for cleanliness/safety. 4 C.S.I. Investigation Report Investigator________________________ TABLE 1: Physical Properties of the 5 Substances Substances Aspirin (C9H8O4) State Physical Properties Colour Clarity Crystal Form Solubility Buffered Aspirin Baking Soda Powdered Sugar (C6H12O6) Sodium Dihydrogen Phosphate (NaH2PO4) Buffered Aspirin (regular aspirin (C9H8O4) mixed with some CaCO3) Baking Soda (NaHCO3) TABLE 2: Chemical Changes of the 5 Substances Aspirin Powdered Sugar Sodium Dihydrogen Phosphate Universal Indicator HCl Fe(NO3)3 I2 5 MYSTERY POWDER LETTER ___________ Now that you’ve carried out these tests, it is your responsibility to identify the mystery powder and determine whether or not it could have been the cause of death. When you study the physical and chemical properties of the mystery powder, you observe the following: Mystery Powder Physical Properties Mystery Powder Chemical Changes State Universal Indicator Colour HCl Clarity Fe(NO3)3 Crystal Form I2 Solubility 1. Based upon these results and your original data, the mystery solid at the crime scene was ________________________. (1) 2. Which physical and/or chemical property(s) allowed you to confirm the identity of the mystery powder? Explain and be very specific by referring to your data. (2) 3. Based on the identity of the mystery powder, I ____________________ (fill in your name), the lead C.S.I. on this case, __________ (do or do not) believe that the powder was the cause of death in this case. My reasons are as follows:(3) 6