Problem 08- Lymphadenopathy
advertisement

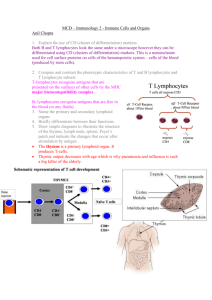
Lymphadenopathy Functions of lymphatic system: One way system returns lymph fluid to the cardiovascular system via vessels. Responsible for the removal of interstitial fluid from tissues returning it back to blood Absorbs and transports fatty acids and fats as chyle from the digestive system Transports white blood cells to and from the lymph nodes into the bones The lymph transports antigen-presenting cells (APCs), such as dendritic cells, to the lymph nodes where an immune response is stimulated. Lymph: Fluid derived from blood plasma. Pushed out through the capillary wall by pressure exerted by the heart or by osmotic pressure at the cellular level. Contains nutrients, oxygen, and hormones, as well as toxins and cellular waste products generated by the cells. Lacteals are lymph vessels that transport intestinal fat and are localized to the GI tract. Lymphatic vessels: Blind-ended tubes with thin endothelial walls (only a single cell in thickness). Primary lymphoid organs = Thymus and bone marrow (generate lymphocytes from immature progenitor cells) Secondary lymphoid organs = Maintain mature naive lymphocytes and initiate an adaptive immune response. Site of antigen activation of lymphocytes e.g peyers patches, spleen, adenoids (Mucosal-associated lymphatic tissue MALT) The spleen: Surrounded by a connective tissue capsule that extends inward to divide the organ into lobules. 2 types of tissue known as red and white pulp: Red pulp consists of venous sinuses filled with blood and cords of lymphocytes and macrophages; white pulp is lymphatic tissue consisting of lymphocytes around the arteries. Lymphocytes are densely packed within the cortex of the spleen. It serves as a reservoir of lymphocytes for the body It filters blood It plays an important role in red blood cell and iron metabolism through macrophage phagocytosis of old and damaged red blood cells It recycles iron by sending it to the liver It serves as a storage reservoir for blood It contains T lymphocytes and B lymphocytes for immunologic response Immunity The immune system is typically divided into two categories--innate and adaptive--although these distinctions are not mutually exclusive. Innate immunity Innate immunity refers to nonspecific defense mechanisms that come into play immediately or within hours of an antigen's appearance in the body. These mechanisms include physical barriers such as skin, chemicals in the blood, and immune system cells that attack foreign cells in the body. The innate immune response is activated by chemical properties of the antigen. Adaptive immunity Adaptive immunity refers to antigen-specific immune response. The adaptive immune response is more complex than the innate. The antigen first must be processed and recognized. Once an antigen has been recognized, the adaptive immune system creates an army of immune cells specifically designed to attack that antigen. Adaptive immunity also includes a "memory" that makes future responses against a specific antigen more efficient. Adaptive Immunity: Humoral and Cell mediated Humoral Immunity The principal functions of B-cells are to make antibodies (immunoglobulins) against antigens, to perform the role of antigen-presenting cells (APCs), and to develop into memory B cells after activation by antigen interaction. The human body makes millions of different types of B cells each day that circulate in the blood and lymphatic system performing the role of immune surveillance. They do not produce antibodies until they become fully activated. Each B cell has a unique receptor protein (referred to as the B cell receptor (BCR)) on its surface that will bind to one particular antigen. The BCR is a membranebound immunoglobulin, and it is this molecule that allows the distinction of B cells from other types of lymphocyte, as well as being the main protein involved in B cell activation. Once a B cell encounters its cognate antigen and receives an additional signal from a T helper cell, it can further differentiate into one of the two types of B cells (plasma B cells and memory B cells) Cell-mediated immunity Most effective in removing virus-infected cells, but also participates in defending against fungi, protozoans, cancers, and intracellular bacteria. It also plays a major role in transplant rejection. Activation of phagocytes, natural killer cells (NK), antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen. CD4 cells or helper T cells provide protection against different pathogens. T Cells mature in the Thymus Cytotoxic T cells cause death by apoptosis without using cytokines, therefore in cell mediated immunity cytokines are not always present. Cellular immunity protects the body by: 1. activating antigen-specific cytotoxic T-lymphocytes that are able to induce apoptosis in body cells displaying epitopes of foreign antigen on their surface, such as virus-infected cells, cells with intracellular bacteria, and cancer cells displaying tumor antigens; 2. activating macrophages and natural killer cells, enabling them to destroy pathogens; and 3. stimulating cells to secrete a variety of cytokines that influence the function of other cells involved in adaptive immune responses and innate immune responses. Cell-mediated immunity is directed primarily at microbes that survive in phagocytes and microbes that infect non-phagocytic cells. It is Characteristic % Ig in plasma Location IgG 80 Inta/extravasc ular IgM 6 Intravascular IgA 13 Secretions/int ravascular IgD <1 B-cell surface Structure Protein subunits Half-life (days) Special features Monomeric Non Pentameric J Dimeric J and S Monomeric None IgE <1 Mast cells, basophils, secretion Monomeric None 23 5 6 3 2 Placental passage Primary antibody produced Present in milk and secretions B cell receptor Mediates atopy Localised Local infection Pyogenic e.g tonsillitis TB Lymphoma Secondary carcinoma Generalised Infection EBV CMV Toxoplasma sp. TB HIV infection Lymphoma Leukaemia Systemic disease SLE Sarcoidosis Rheumatoid arthritis Drug reaction e.g. phenytoin Causes of lymphadenopathy Differential diagnosis Subcutaneous lesions, eg lipoma, abscess Hernia Skin lesions, eg sebaceous cyst Neck: branchial cleft cysts, cystic hygromas, salivary glands, thyroglossal duct cysts (usually in midline) In the absence of obvious infection or any other underlying disorder, or if there is any suspicion of malignancy - refer. Localised lymphadenopathy should prompt a search for an adjacent precipitating lesion and an examination of other nodal areas to rule out generalised lymphadenopathy. Lymph nodes greater than 1 cm in diameter are generally considered to be abnormal. History of malaise and weight loss may be associated with malignancy or certain infections, eg HIV, TB. Painful, tender lymph nodes are usually associated with infection. Firm or hard, painless nodes are often associated with malignancy. Full examination is essential and may reveal associated bruising (eg leukaemia) orhepatosplenomegaly (eg lymphoma). Lymphadenopathy in the neck may cause superior vena cava obstruction. Investigations o o o o o o Full blood count: white cell count raised in infection or malignancy, blood film for leukaemia Acute phase reactants, eg ESR and CRP, often raised in infection or malignancy Liver function tests: liver infiltration Infection: Swabs from primary infection site for culture and sensitivities Viral titres, eg Epstein-Barr virus, HIV, hepatitis Investigations for TB Syphilis serology Toxoplasma screen Blood cultures Autoantibody screen: SLE, rheumatoid arthritis Chest X-ray: sarcoidosis, TB, primary or secondary malignancy CT scan: nodal distribution, staging of lymphoma Fine needle aspiration or biopsy (ideally excisional biopsy) of lymph node may be required; some patients, particularly children, remain undiagnosed after biopsy o o o Sentinel node biopsy: The sentinel node is the first node identified or the node with the highest radioactivity count. Sentinel node biopsy can avoid the need for block dissection of lymph nodes. It is often used in the preoperative assessment of breast cancer and melanoma and may have benefits in other cancers. The sentinel node can be identified either by local injection of blue dye and following its path, or by gamma camera imaging following injection of 99mTc Leukaemia Malignant neoplasm of haematopoietic stem cells, characterised by diffuse replacement of the bone marrow by neoplastic cells. In most cases cells spill over into blood where they can be seen in large numbers. Cells may also infiltrate liver, spleen, lymph nodes and other tissues. Overall incidence 10/100,000 Leukaemia can be divided on the basis of the speed of evolution of the disease (acute or chronic) and further subdivided upon cell type Acute myeloid leukaemia (AML) Acute lymphoblastic leukaemia (ALL) Chronic myeloid leukaemia (CML) Chronic lymphocytic leukaemia (CLL) Aetiology Most cases unknown. Genetic: More common in some chromosomal disorders (e.g. downs) Philadelphia chromosome. Found in 95% CML and some ALL. Long arm of chromosome 22 shortered by reciprocal translocation to long arm of chromosome 9 (t(9,22)) Environmental: Chemicals e.g. benzene compunds in industry Drugs e.g AML after treatment with alkylating agents e.g. mephalan Radiation exposue. Can iduce genetic damage to haematopeitic precursors, increased incidence of Nagasaki and Hiroshima survivors and patients treated with ionising radiation. Acute leukaemia Clonal proliferation of myeloid or lymphoid precursors. Reduced capacity to differentiate into mature cellular elements. ↑Leukaemic cells in bone marrow, peripheral blood and other tissues. ↓ Red cells, platelets and neutrophils Epidemiology Both types can occur at any age but generally: AML Middle aged-elderly ALL Childhood Clinical features Anaemia Bleeding Infection (e.g. pneumonia) Peripheral lymphadenopathy Splenomegaly Investigations Peripheral blood film and bone marrow aspirate FBC: anaemia and thrombocytopaenia (NB WCC usually raised but may be normal or low) If fever: Chest xray and blood cultures Bone marrow: Increased cellularity, high % abnormal lymphoid or myeloid blast cells AML 1. AML with recurrent cytogenic abnormalities (includes acute polymyelocytic leukaemia with t(15;17) or variants 2. AML with multilineage dysplasia 3. AML and MDS, therapy related, occurring after chemotherapy or radiotherapy 4. AML-not otherwise categorised ALL 1. Precursor B cell ALL 2. Burkitt cell leukaemia 3. Precursor T cell ALL MDS, myelodysplastic syndromes Management Induction chemotherapy (returns blood film and bonemarrow to normal) Risk of failure based on cytogenic pattern Successful remission induction followed by further treatment (consolidation) Supportive care: Blood transfusion and platelets. Treat infection with IV antibiotics. Prevent acute tumour lysis syndrome (massive and rapid breakdown tumour cells leads to increased urate, potassium phosphate and hypocalcaemia. AKI due to deposition in renal tubules. Prevent and treat with allopurinol, urate oxidase and high IV fluids) Treatment of AML Treat with curative intent (<60years) Low risk of failure (based on cytogenic pattern) o IV chemo. E.g. cytabarbine and danorubicin Intermediate risk o Consolidating chemotherapy to induce remission o Bone marrow transplant (sibling matched, allogenic) High risk o Only curable with allonegnic transplant. NB. High risk associated with advanced age, when toxicity of treatment increases. Prognosis: 30% cure rate Complete remission in 75% of under 60 year olds. 50% of those entering remission will be cured. Long survival after recurrence rarely achieved without allogenic transplant. Treatment of ALL Remission induction with combination chemo e.g. vincristine, dexamethasone, asparginase and danorubicin. Propensity to involve CNS, so prophylactic intrathecal drugs e.g. methotrexate Prognosis: Good, 80% alive and disease free at 5 years, almost all achieve remission. Worsens with age. Chronic myeloid leukaemia Middle age Philadelphia chromosome Insidious onset: Fever Weight loss Sweating Symptoms of anaemia Splenomegaly Untreated chronic phase lasts 3-4 years. Followed by blast transformation with development of acute leukaemia (usually AML) and commonly, death. Investigations FBC: Anaemia + raised WCC (NB> platelets may be low, normal or raised) Bone marrow aspirate: hypercellular marrow with increase in myeloid progenitors. Philadelphia chromosome and BCR-ABL oncogene shpwn by cytogenics and reverse transcriptase PCR. Management Imatinib, for chronic phase. Blocks anzymatic action of BCR-ABL fusion protein. Complete haematological response in 95%. In acute phase: chemotherapy, as of acute leukaemia to attempt to induce a second chronic phase. Chronic lymphocytic leukaemia Incurable disease of older people. Characterised by uncontrolled proliferation and accumulation of B lymphocytes (although T cell CLL does occur) Clinical features Early asymptomatic . Symptoms of bone marrow failure: anaemia, bleeding, infection. Autoimmune haemolysis in some patients Lymphademopathy Hepatosplenomegaly Investigations FBC: Raised WCC and lymphoctyosis (+anaemia, thormobocytopaemia) Blood film: Small lymphocytes of mature appearance (smear/smudge cells-an arterfact of cell rupture while film being made) Bone marrow: Lymphocyte infiltration Immunophenotyping: Excludes reactive lymphocytosis and other lymphoid neoplasms Management Early stage: Expectant Indictaions for treatment=advanced stage or symptoms e.g. anaemia, infections, splenic discomfort Oral chlorambucil(+/- prednisolone) palliates. Fludarabine, cyclophosphamide and/or rituximab has an impact on bone marrow and can induce remission. Prognosis Median survival variable. Patients with 11q or 17p deletions (sites of two tumour suppressor genes) at high risk of not responding to treatment. Lymphoma Neoplastic transformations of normal T or B cells which reside predominantly in lymphoid tissues. Commoner than leukaemia. Histological appearance classifies into Hodgkins and Non-Hodgkins Hodgkins lymphoma Primarily disease of young adults Previous EBV infection may have a role in pathogenesis Clinical features Painless lymphnode enlargement (cervical) ‘rubbery’ consistency Hepatosplenomegaly Systemic ‘B’ symptoms= fever, drenching night sweats, weight loss (>10% 6 months) Other=pruritis, fatigue, anorexia, alcohol induced pain at site of enlarged lymph nodes Invetsigations FBC: May have normocytic, normochromic anaemia Raised ESR LFTs LDH is an adverse prognostic marker CXR- mediastinal widening from enlarged nodes Lymphnode biopsy: REED-STEINBERG CELLS (malignant B lymphocytes in a background of benign small lymphocytes and histiocytes) CT-disease staging Management Stage 1 – Single lymph node region or single extra lymphatic organ or site Stage 2 - 2 or more groups of lymph nodes affected. same side of diaphragm Stage 3 – Lymph nodes affected on both sides of the diaphragm Stage 4 – Diffuse or disseminated involvement of one or more extra lymphatic organs or tissues, with or without associated lymph node involvement