Stefanie Spayd & Janet Fang
Dynamics of Climate Change and Climate Variability
Drs. Lisa Goddard and Mark Cane
December 10, 2006
The Carbon Cycle
The carbon cycle is the natural cycling process by which
carbon is exchanged between the Earth’s biosphere, lithosphere, hydrosphere, and
atmosphere. This hierarchy of subcycles operates on different time scales, ranging from
the relatively short replenishing of CO2 in the atmosphere, to
the relatively long recycling of carbon through sedimentary
rocks.
In the past, humans were a minute part of the cycle, as a
contributor to the cycle as we exhale and when our bodies
decay after we die. More recently, particularly since the
industrial revolution, humans have begun to contribute to the
cycle in a way that the rest of the cycle cannot keep up with.
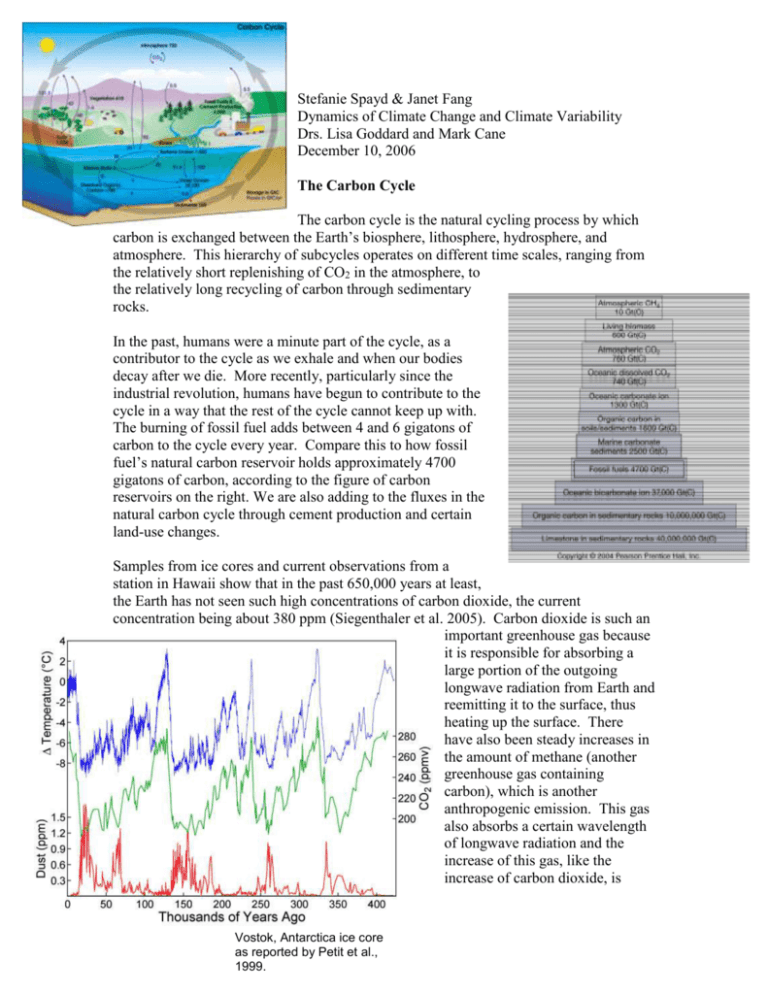
The burning of fossil fuel adds between 4 and 6 gigatons of
carbon to the cycle every year. Compare this to how fossil
fuel’s natural carbon reservoir holds approximately 4700
gigatons of carbon, according to the figure of carbon
reservoirs on the right. We are also adding to the fluxes in the
natural carbon cycle through cement production and certain
land-use changes.
Samples from ice cores and current observations from a
station in Hawaii show that in the past 650,000 years at least,
the Earth has not seen such high concentrations of carbon dioxide, the current
concentration being about 380 ppm (Siegenthaler et al. 2005). Carbon dioxide is such an
important greenhouse gas because
it is responsible for absorbing a
large portion of the outgoing
longwave radiation from Earth and
reemitting it to the surface, thus
heating up the surface. There
have also been steady increases in
the amount of methane (another
greenhouse gas containing
carbon), which is another
anthropogenic emission. This gas
also absorbs a certain wavelength
of longwave radiation and the
increase of this gas, like the
increase of carbon dioxide, is
Vostok, Antarctica ice core
as reported by Petit et al.,
1999.
threatening to close the
atmospheric window, which is an
area where longwave radiation in
the atmosphere can escape out to
space, creating a radiation
imbalance. If this window closes
due to increased absorption from
an excess of greenhouse gases in
the atmosphere, the Earth will
continue to warm, even after the
gases stop being emitted (Kump
et al. 2004). The increases in
http://science.hq.nasa.gov/oceans/system/carbon.html (NASA 2005)
methane can be attributed to
several anthropogenic causes, for
example, forest fires, cattle, and rice paddies. Methane is also released when organic
materials decay. If humans continue to contribute to these greenhouse gases in the same
business as usual amounts, there is an expected increase in temperature anywhere from
1.4 to 5.8 degrees Celsius, depending on which model you look at (IPCC TAR 2001).
Source: IPCC Climate change 2001 - Synthesis report
Earth’s carbon budget is determined by atmospheric CO2 concentration, fossil fuel CO2
emissions, the net terrestrial CO2 fluxes (which include CO2 fertilization and land-use
change), the net oceanic uptake of CO2, and some other residual CO2 fluxes.
2.123
C
S fossil Sterrestrial S ocean S residual
t
Carbon dioxide is absorbed by the oceans – anthropogenic emissions in particular, in
what is known as the solubility pump, or the oceanic “conveyor belt.” The solubility
pump removes carbon from the atmosphere; anthropogenically carbon dioxide-laden air
mixes with and then dissolves into the surface layer of the ocean (NASA 2005). The
solubility pump removes approximately 30% of the anthropogenic CO2. Carbon dioxide
is more easily dissolved, or more soluble, in cold water, so at high latitudes where surface
cooling occurs, carbon dioxide-loaded water sinks to the deep ocean and becomes part of
the deep ocean circulation “conveyor belt”, where it stays for hundreds of years.
Eventually, mixing of the deep and shallow layers brings the water back to the surface at
the opposite end of the conveyor belt in regions distant from where the carbon dioxide
was first absorbed, generally in the tropics. In the tropical regions, warm waters cannot
retain as much carbon dioxide and so the carbon dioxide is transferred back into the
atmosphere (NASA 2005).
Another mechanism of the ocean in which
carbon is absorbed is the carbonate, or
inorganic, pump. This is a very important
area of absorption. As the ocean takes in
more carbon, it becomes more acidic.
Carbonic acid forms, along with
bicarbonate. As these molecules are
increasing, creating a more acidic oceanic
water, the basic molecules of the ocean, like
carbonate are becoming less and less. Many
tiny organisms, or nanoplankton, including
Coccolithophores, use calcium carbonate to
make themselves protective exoskeleton
shells. Coccoliths, which are the little
shields that make up the protective shell
layer for a Coccolithophore, are the
exoskeleton for these important, but tiny creatures. Recent experiments have shown that
a decrease in the pH of water (making it more acidic) leads to a disintegration of these
shells. There are many important factors related to nanoplankton: they are at the top of a
large oceanic food chain, their blooms create albedo (reflecting sunlight from the Earth’s
surface), and they are part of the oceanic carbon sink. The disintegration of their shells is
likely to result in the death of these nanoplankton because they become more vulnerable
prey as they are no longer protected. If they die out, then there is a loss at the very top of
the food chain, causing deaths then in the rest of the food chain below them. Also, during
what are known as blooms of
nanoplankton when their
populations increase and they
are growing,
Coccolithophores create a
white, milky color on the
surface of the ocean, which is
so large that it actually
increases the albedo of the
Earth, reflecting sunlight out into the atmosphere. If many of the nanoplankton species
die out, the Earth will lose precious albedo power, thus allowing the temperature of the
planet to increase (Ruttiman 2006).
The last of the ocean carbon pumps is the organic pump: oxygen production in shallow
waters through (1) photosynthesis and (2) the settling down of organic matter from fecalpellet production, is combined with oxygen consumption in deep waters through (1)
decomposition and (2) nutrient release. The overall effect of this is the transfer of CO2
and nutrients between surface waters and the deep ocean.
Terrestrial sinks are also where a lot of carbon is stored, taken out from the atmosphere,
until chemical processes can remove it again. One of the greatest terrestrial carbon sinks
are young forests. A hectare of trees holds up to fifty times more carbon than a hectare of
crops or grasses (Houghton 2002). When trees are cut down and used as timber, the
carbon goes with them, and can be stored in our houses and buildings. It is good that
carbon can be trapped this way,
but the cutting of trees then
reduces the amount that can be
taken up in the terrestrial
biosphere unless relatively mature
trees are planted in their place.
Because of the seasonality of the
Earth, the amount of carbon in the
atmosphere varies with the
seasons. This can be witnessed in
the graph of atmospheric carbon
concentrations taken from Mauna
Loa, Hawaii. As new trees and
flowers are sprouting in the
Northern Hemisphere’s spring
and summer months, carbon
dioxide is taken out of the
atmosphere for photosynthesis by
the plants. There is not the same
change noticed in the spring and summer of the Southern Hemisphere because there is
more land area in the Northern Hemisphere than in the Southern Hemisphere, so global
atmospheric carbon increases during these months (Quay 2002).
There is a lot of uncertainty about
the future of the carbon cycle,
especially the effect that it will
have on the temperature of the
Earth. Past models show a strong
correlation between temperature
and carbon dioxide, and, although
under natural variability it appears
that the Earth warms before
carbon dioxide increases,
scientists still find this a
worrisome trend. The Earth is
currently warming, and the
concentrations of carbon dioxide
are also increasing. What is
unclear is how much warmer the
Earth will get and when the
amount of carbon dioxide and
methane we have already emitted
will become stable again with the
rest of the carbon cycle.
Shindell 1998
Research is continuing in a technologically improving world. As more information
surfaces through direct measurements, there will be less uncertainty about the future and
the ways to model the close relationship of the carbon cycle to the atmosphere. It is
important to understand that carbon affects other Earth systems, including the biosphere,
lithosphere, hydrosphere, and atmosphere, whether directly or indirectly, and that carbon
has the power to change the future. (This paragraph doesn’t really add anything, and
cou
ld
be
dro
ppe
d)
What you should take home from this research is that:
(1) Humans are affecting the carbon cycle, which has the potential to change the
climate of the Earth. A changing climate will create other changes that may
ultimately throw off entire ecosystems, further changing the climate.
(2) There are different ways in which carbon is stored and budgeted in sinks and the
ways that carbon moves between these sinks through chemical and dynamical
processes. If you remove a sink or a means of movement, the entire system has
the potential to change itself and change other Earth systems.
(3) There is uncertainty about the future of the carbon cycle, especially around the
question of its effect on the future temperature of the planet. This is primarily
because the link between carbon and temperature is not fully understood; there is
evidence to support the link, but more research is necessary to create better
models with less uncertainties. With more data collected from direct
measurements instead of from data that has a lot of uncertainties, like ice core
samples, models can be improved to show a more accurate account of the future
of carbon, specifically in our atmosphere.
References and Sources for Further Information
Dennis Baldocchi (2005) The carbon cycle under stress. Nature 437:483-484.
Ken Caldeira and Michael E. Wickett (2003) Nature 425:365.
P. Falkowski et al. (2000) The Global Carbon Cycle: A Test of Our Knowledge of Earth
as a System. Science 290:291-296.
Houghton, R.A. Terrestrial carbon sinks—uncertain explanations. Biologist (2002) 49
(4) 156-160.
Lee R. Kump, James F. Kasting, and Robert G. Crane (2004) The Earth System, 2nd
Edition. Pearson Prentice-Hall, Upper Saddle River, New Jersey.
James C. Orr et al. (2005) Anthropogenic ocean acidification over the twenty-first
century and its impact on calcifying organisms. Nature 437:681-686.
Quay, Paul. Ups and downs of CO2 uptake. Science (2002) 298 2344.
Jacqueline Ruttimann (2006) News Feature: Sick Seas. Nature 442:978-980.
Urs Siegenthaler, et al. Stable Carbon Cycle-Climate Relationship During the Late
Pleistocene. Science (2005) 310 1313-1317.
IPCC Third Assessment Report, accessed at http://www.grida.no/climate/ipcc%5Ftar/.