#1 GROSS BRAIN

#1 GROSS BRAIN
Brains for study are those which were removed during gross dissection and stored in 10% formalin.
A. Meninges - In most specimens the dura has been removed, totally or in part. The information is here for review. Meninges will be seen on MRI scans and on sections of the spinal cord.
1. The central nervous system is surrounded by three connective tissue membranes; the dura mater, the arachnoid, and the pia mater.
2. The brain and spinal cord are derivatives of neuroectoderm; however, the dura arises from condensations of paraxial mesoderm. Neural crest cells give rise to the piaarachnoid (or leptomeninges).
3. The dura mater ( NN42 ) is the outermost meninx and consists of two layers of dense, fibrous connective tissue. The outer layer of the dura adheres tightly to the overlying bone and forms the periosteum of the skull. The inner layer (true dura) makes folds such as the falx cerebri ( NN42 ) and the tentorium cerebelli ( NN42 ). The two layers of the dura frequently separate to form the venous sinuses which drain the brain.
4. Because of its close attachment to the skull, most of the dura is usually left in the cadaver. The meninx immediately internal to the dura is the arachnoid ( NN42 ). It is a delicate, nonvascular membrane separated from the dura by the potential subdural space ( NN42 ). Ruptured veins may hemorrhage into this space, putting pressure on the brain and this pressure, in turn, may compromise the blood supply to the brain.
Web-like extensions of the arachnoid traverse the subarachnoid space ( NN42 ) to join the pia. The subarachnoid space, in life, is filled with cerebrospinal fluid. The pia mater ( NN42 ) is a thin, vascularized connective tissue layer which closely follows the contours of the central nervous system and is adherent to this underlying tissue.
The pia mater forms the tunica adventitia of the blood vessels and passes into the nervous tissue with these vessels for a short distance. The meninges invest cranial and spinal nerves as they emerge from the central nervous system.
B. Cisterns (Some of the following may be seen in H2-29 , H2-30 , but they are not labeled).
1. Cerebrospinal fluid enters the subarachnoid space via three foramina in the medulla which will be described in lecture. Expansions, or cisterns ( H2-29 ), in the subarachnoid space can be seen on your specimen. The spaces are formed when the arachnoid does not follow the contours of the nervous tissue. Since arachnoid has been removed from specimens, you will have to look for areas where arachnoid can bridge across crevasses and thereby create cisterns. Identify the major cisterns on your specimen: cisterna magna ( H2-29 ), (between the cerebellum and the medulla), pontine cistern ( H2-29 ), on the ventral surface of the brainstem at the junction of the pons and the medulla, interpeduncular cistern ( H2-29 ) between the two cerebral peduncles, and the quadrigeminal cistern ( H2-29 ) related to the colliculi which are also known as quadrigeminal bodies. The midline foramen of
Magendie ( H2-29 ) is a communication between the fourth ventricle and the cisterna magna . The lateral foramina of Luschka ( H2-29 ) connect the fourth ventricle with the pontine cistern .
2. Arachnoid villi ( NN42 ) are the major areas of transfer of the cerebrospinal fluid back to the blood. Note that these villi are bathed in the blood contained within the venous sinuses. In older individuals these villi become hypertrophied and become arachnoid granulations ( NN42 ).
C. External Form of the Central Nervous System
The Brain. Labeled photographs of the structures on the gross brain you will encounter in this exercise and in subsequent laboratory exercises are found in Chapter 2, pages 16-36, in the atlas.
1. Brain Stem and Cerebellum (Use the whole brain and the brain stem for the following observations): a. Medulla Oblongata (H2-17, H2-20, H2-22, H2-23, H2-34, H2-36)
(1) Identify the medulla ( H2-17 ), the most caudal part of the brain stem.
On the lateral surface identify the olive ( H2-17 ), relay center from telecephalon to cerebellum. In the anterolateral sulcus ( H2-17 ), a shallow groove just anterior to the olive, you may find some root filaments of the hypoglossal nerve ( H2-17 ). In the posterolateral sulcus ( H2-17 ), behind the olive, look for root filaments of the glossopharyngeal and vagus nerves. b. c.
(2) Observe the dorsal surface of the cerebellum ( H2-31 ) and note that it consists of two lateral hemispheres ( H2-31 ) and a midline vermis
( H2-31 ). Note that the surface of the cerebellum is thrown into a number of transverse folds or folia ( H2-31 ).
Pons
(1) The pons ( H2-20 ) is continuous with the rostral end of the medulla.
Ventrally, it is formed by a broad band of fibers which continues dorsally on each side as the middle cerebellar peduncle ( H2-20 ).
Identify the roots of the trigeminal nerve ( H2-20 ) emerging from the anterolateral surface of the pons.
The abducens nerve ( H2-20 ) may be seen emerging anteriorly near the midline between the pons and medulla. Look for the facial nerve ( H2-20 ) and vestibulo-cochlear nerves ( H2-20 ) emerging at the junction of the pons, medulla and cerebellum, the cerebello-pontomedullary angle.
Mesencephalon (midbrain)
(1) On the ventral side of the brain stem ( H2-23 ), identify the cerebral peduncles, ( H2-23 ), labeled crus cerebri, efferent and afferent connections with the cerebral hemispheres, and the oculomotor nerve ( H2-20 ) emerging from the fossa between cerebral peduncles, the interpeduncular fossa ( H2-20 ). On the dorsal surface of the mesencephalon ( H2-34 ) identify the superior colliculus ( H2-34 ), a
2. reflex center for sight, and the inferior colliculus ( H2-34 ), a reflex center for hearing.
Diencephalon a. b.
The diencephalon ( H2-29 ) is located between the telencephalon and the mesencephalon. It is overlapped dorsally by the expanded cerebral hemispheres from which it is separated by the transverse cerebral fissure.
Ventrally locate a pair of protuberances, the mammillary bodies ( H2-20 ) which form the caudal portion of the hypothalamus, rostral to which the infundibular stalk ( H2-23 ) of the pituitary gland is attached. Still more rostrally are the optic chiasm ( H2-23 ), the crossed portions of the optic nerves, and the optic tracts which terminate in lateral swellings, the lateral geniculate bodies ( H2-20 ) which are hidden by the overlapping cerebrum.
(The atlas does not have a picture of the termination of the optic tract, but they can be seen on the brain stems.) The optic nerves ( H2-20 ) are technically part of the diencephalon.
3. Telencephalon a. b.
Location. The telecephalon develops from the rostral terminal portion of the primitive neural tube. Its medial portion is rostral to and continuous with the diencephalon. Due to extensive overgrowth, its cerebral hemispheres extend rostrally, laterally and caudally and overlap the diencephalon.
General Description. The hemispheres are separated one from the other by the longitudinal cerebral fissure ( H2-8 ) in which the falx cerebri would be located and from the diencephalon, mesencephalon and cerebellum by the transverse cerebral fissure ( H2-11 ). c. The Subdivisions of the Cerebral Cortex. In development, the cerebral cortex expands out of proportion to the underlying structure and is consequently thrown into a series of convolutions or gyri ( H2-8 ) separated from one another by shallow sulci ( H2-11 ) or deeper fissures . These fissures and sulci divide the cortex into lobes ( H2-5 ) each of which is subdivided into a series of gyri which are more or less constant from individual to individual.
Identify the lateral sulcus (Sylvian fissure) ( H2-11 ) and central sulcus
(Rolandic sulcus) ( H2-11 ) and determine the extent of the four lobes of the cerebrum.
(1) The frontal lobe ( H2-11 ) is the anterior portion of the hemisphere, terminating in the frontal pole. It is limited posteriorly by the central sulcus ( H2-11 ) and inferiorly by the lateral cerebral sulcus ( H2-11 ).
Its most posterior gyrus, the precentral gyrus ( H2-11 ), contains a major motor control center.
(2) The occipital lobe ( H2-11 ) includes the occipital pole of the hemisphere. Anteriorly it is limited on its medial surface by the parieto-occipital sulcus ( H2-29 ), which is best seen on the medial
4. surface and cuts the superior margin of the hemisphere. It is limited on the lateral surface by a line drawn between the superior end of the parieto-occipital sulcus and the preoccipital notch ( H2-11 ). The visual receptive center is located in the extreme posterior part of the lobe.
(3) The temporal lobe ( H2-11 ) is bounded superiorly by the lateral sulcus and a line from the posterior end of this sulcus to the anterior border of the occipital lobe ( H2-11 ). It terminates in the temporal pole of the hemisphere.
(5) The insula ( H2-46 ) is an island of cortex in the depths of the lateral cerebral sulcus. It is completely hidden by the overgrowth of the temporal, frontal, and parietal lobes. The covering flaps of the lobes are called the operculum ( H2-46 ).
(6) The areas of functional localization in the cerebral cortex will be the focus of future study. Locate the central sulcus and lateral sulcus as projected on the external surface of the head.
Cranial Nerves a.
(4) The parietal lobe ( H2-11 ) is located between the frontal and occipital lobes superior to the temporal lobe. Its most anterior gyrus, the postcentral gyrus ( H2-11 ), is the somesthetic sensory area, or common sensory projection center.
Because a major part of the study of neuroanatomy is concerned with the structure and function of the cranial nerves, it is important to obtain a thorough knowledge of these structures. They are shown in H2-17 , H2-20 ,
H2-22 and depicted on the following diagram of the brain stem and diencephalon ( Fig. 1 ). b. You should try to locate all of the cranial nerves ( H2-20 ) except the olfactory nerve. Details of their central connections will be studied subsequently.
(1) Olfactory - enters the olfactory bulb on the inferior surface of the frontal lobe. Sometimes fine fila can be seen
(2) Optic -
(3) Oculomotor - attached to the olfactory bulb. enters diencephalon. exits from interpeduncular fossa of midbrain.
Not present on all brain stems.
(4) Trochlear - the only nerve which exits dorsally. Not present on all specimens. If present, it can be seen on the superior medullary velum, the roof of the fourth ventricle.
(5) Trigeminal -
(6) Abducens - emerges from the lateral aspect of pons. emerges at the junction between the pons and medulla near the midline.
(7) Facial - emerges from the cerebellopontomedullary angle.
c.
(8) Vestibular cochlear - emerges from the cerebellopontomedullary angle.
(9) Glossopharyngeal - emerges from the posterolateral sulcus of the
(10) Vagus - medulla, just posterior to the inferior olive. emerges from the posterolateral sulcus of the medulla, just posterior to the inferior olive.
(11) Spinal Accessory - If a cranial division is postulated, the fibers will emerge in the same sulcus as that used by IX and X.
Spinal fibers emerge laterally from the upper 3-4 segments of the spinal cord.
(12) Hypoglossal - emerges from the anterolateral sulcus of medulla, anterior to the inferior olive.
Nerves are composed of various types of fibers which may be afferent
(sensory) or efferent (motor) or may be associated with the soma or the viscera. Below is a list of the types of fibers which occur in cranial nerves.
(1) General Somatic Afferent (GSA) - convey afferent impulses from the general soma such as the skin, bone, skeletal muscle, joints, ligaments,
& tendons
(2) General Visceral Afferent (GA) - afferent fibers from the visceral receptors like carotid body, carotid sinus
(3) Special Afferent (SA) - afferent fibers from the chemical receptors of taste and smell and those associated with sight and receptors of the inner ear.
(4) General Somatic Efferent (GSE) - efferent fibers innervating skeletal muscle of nonbranchiomeric origin.
(5) General Visceral Efferent (GVE) - fibers belonging to the autonomic nervous system and innervating smooth
(6) Branchial Efferent (BE) - and cardiac muscles and glands. efferent fibers supplying the branchial arch muscles.
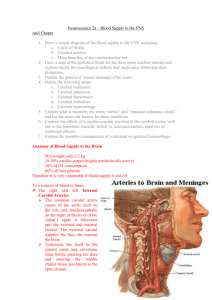
5. Vascular Supply a. Arterial Supply to the Brain
(1) Impairment of blood supply to the brain often resulting in necrosis and degeneration represents the most common injury to this tissue. It is important to visualize the major vessels supplying areas of the brain because functional impairment can frequently be related to inadequate blood supply to that area which controls a particular function.
(2) Two pairs of arteries supply the brain, the two internal carotid arteries ( H2-21 ) and the two vertebral arteries ( H2-21 ). The internal carotid artery, prior to its position as seen on the brain, has passed through the cavernous sinus with cranial nerves III, IV, VI ( N98 ) and the ophthalmic and maxillary branches of V ( N98 ). (An infection or lesion of this area can have far reaching neurological effects). After leaving the cavernous sinus, the internal carotid artery usually proceeds upward and backward although there may be some
variations to this pattern.
(3) The vertebral artery ( H2-21 ) is the first branch of the subclavian and ascends through the transverse foramina of the upper six cervical vertebrae to enter the cranial cavity via the foramen magnum.
Examine the ventral surface of the brain and identify the two vertebral arteries ( H2-21 , H2-24 ). At the lower part of the medulla (before the two vertebrals unite) each gives off a posterior spinal artery ( H2-24 ) that proceeds down the spinal cord on its posterior surface. The posterior spinal a. may arise from the posterior inf. cerebellar. A medial branch arises from each vertebral, the branches unite on the ventral surface of the medulla to form the anterior spinal artery ( H2-
21 ). Close to the origins of these spinal branches, identify the laterally projecting posterior inferior cerebellar arteries ( H2-21 ), in many specimens these arteries may have been left in the body. The two vertebral arteries unite to form the basilar artery ( H2-21 ) which lies on the ventral surface of the pons. Compare your gross specimen with the atlas and identify the anterior inferior cerebellar artery ( H2-21 ), labyrinthine artery (the labyrinthine artery is usually tiny and would be severed from its attachment because it enters the internal auditory meatus), pontine arteries ( H2-21 ), and the superior cerebellar artery ( H2-21 ). The entire caudal portion of the brain is supplied by branches of the vertebral arteries.
(4) The basilar artery bifurcates to form the posterior cerebral arteries
( H2-21) . These communicate with the internal carotids by means of the posterior communicating arteries ( H2-21 ). The anterior cerebral arteries ( H2-21 ) pass forward from the internal carotids and are connected by the anterior communicating artery ( H2-21 ), completing the cerebral arterial circle (circle of Willis) ( H2-21 ). The other large branch of the internal carotid artery is the middle cerebral artery ( H2-21 ). It reaches the lateral surface of the hemisphere in the lateral cerebral sulcus. Shortly after branching the middle cerebral gives off the anterior choroid artery ( H2-21 ) and lenticulostriate arteries ( H2-21 ) which supply deep structures in the cerebrum.
(5) Review the vessels associated with the cerebral arterial circle and diagram them for your own use. Pay particular attention to the relative caliber of these arteries and remember that the A ideal @ and normal are not synonymous in this case. The A ideal @ circle occurs in only about 25% of specimens. Anomalous origins of vessels, accessory vessels and vessels with very narrow diameters are frequently seen.
(6) The posterior cerebral artery ( H2-15 ) supplies the lower surface of the temporal lobe of the cerebral hemisphere and the medial (and some of the lateral) surface of the occipital lobe. The middle cerebral artery ( H2-12 ) proceeds laterally from its origin to emerge from the lateral sulcus on the lateral side of the brain. This artery supplies most of the lateral part of the cerebral hemisphere. The anterior cerebral artery ( H2-27 ) extends over the medial surface of the hemisphere and also supplies the superior portion of the lateral surface of the frontal and parietal lobes.
6. b.
Neuroglia a. b.
Protoplasmic Astrocyte. Protoplasmic astrocytes ( NH27 ) are found chiefly in the gray matter. These glial cells have processes that are expanded in their tips to form perivascular feet , which wrap around blood vessels in the central nervous system. A blood vessel lies adjacent to this astrocyte. Foot processes are not seen but can be imagined.
Fibrous Astrocyte. Fibrous astrocytes ( NH28 ), or A spider cells, @ have fewer processes than protoplasmic astrocytes but also have perivascular feet as would be seen at the right of this slide if in focus. The processes of these cells are straight and slender. These cells are found in the white matter. c. Oligodendrocyte. Oligodendrocytes ( NH29 ), or oligodendroglia, are found both in the gray and the white matter. They can be seen frequently lined up in rows between myelinated fibers as indicated on this slide. These cells have smaller nuclei than those of astrocytes and they have fewer and shorter processes. Oligodendrocytes form the myelin of fibers in the central nervous system. d.
Venous Drainage of the Brain. ( H2-19 , H2-28 ).
(1) The veins of the brain drain into the dural sinuses. The superior sagittal sinus ( H2-28 ) found in the superior portion of the falx cerebri is sizable because most of the drainage from the cerebral hemisphere is carried to this sinus by superior cerebral veins ( H2-13 ). These veins can be seen on the superior aspect of the hemisphere. The inferior sagittal sinus ( H2-28 ) drains into the straight sinus ( H2-28 ).
The superior sagittal sinus empties directly into the transverse sinus
( H2-28 ), usually the right. The great cerebral vein ( H2-28 ), which drains deep parts of the hemisphere, enters the straight sinus.
(2) The arterial supply and venous drainage of the brain (Chapter 8, pages 240-252) should be studied on the arteriograms or scans mounted on x-ray view boxes in the laboratory.
Microglia. Microglia ( NH30 ) are small, elongate cells that are the smallest of the neuroglial elements. Microglia occur in both gray and white matter and are active in phagocytosis.