Chemical and Physical Change, Qualitative Test for Gases
advertisement

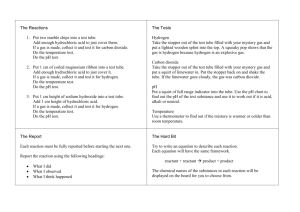
Group Members: Chemical and Physical Change Qualitative Test for Gases Apparatus and Materials: 3% hydrogen peroxide manganese (IV) oxide powder calcium magnesium ribbon copper sulfate (bluestone) goggles at all times stirring rod scoopula straw test tube rack lighter rubber stopper wooden splints test tubes Procedure: Lab Station 4 Demo by teacher: (A) 2-3 mL of sodium chloride solution are added to 2-3mL of silver nitrate solution. (B) A piece of copper wire is place in silver nitrate solution Lab Station 1 1. Fill one test tube about one-third full with water 2. Place a piece of calcium metal into a test tube and immediately cover it with a rubber stopper. Collect the gas produced. 3. When the reaction is over, remove the stopper and immediately insert a burning splint into the test tube. 4. Record observations in chart 5. Dispose the contents down the sink. Lab Station 2 1. Place 5 mL of limewater into a clean test tube. 2. Insert a straw into the limewater then exhale through the straw (Do NOT inhale!) 3. Record observations in chart 4. Dispose the contents down the sink. Lab Station 3 1. Fill a clean and dry test tube with about 5 mL (one thumb width) of hydrogen peroxide. 2. Add to the peroxide a tip of a scoopula of manganese dioxide and lightly place a rubber stopper in the mouth of the test tube. 3. Record observations in chart 4. When the reaction is over, insert a glowing splint into the test tube. 5. Record observations in chart. 6. Dispose of all liquids in the proper waste container! 1. Place about 5 mL of dilute hydrochloric acid in a clean test tube. 2. Add one small piece of magnesium ribbon to the acid and place a rubber stopper in the mouth of the test tube. 3. Record observations in chart 4. When the reaction is over, remove the stopper and immediately insert a burning splint into the test tube. 5. Record observations in chart 6. Dispose of all liquids in the proper waste container! Lab Station 5 1. Add to a clean and dry test tube a tip of a scoopula of copper (II) sulfate 2. Add 5 mL of water to the copper (II) sulfate and gently shake. 3. Record observations in chart 4. When done, dispose of solution in the proper waste container! - Discussion Questions 1. Manganese (IV) oxide acted a s a catalyst in Lab 1. What is a catalyst? What role does it play in a chemical reaction? 2. In Lab 2 an aqueous solution was formed. What is an aqueous solution (aq)? 3. What is a precipitate and how is it formed? Lab Report Due: Group Members: Lab # Starting Substances Physical Properties of starting substances Changes Observed After Mixing (e.g., state, colour change, gas formation etc.) Physical or Chemical Change? Description of results from Gas Test Demo (A) - sodium chloride silver nitrate NA Demo (B) - copper silver nitrate NA 1 2 3 4 5 NA Group Members: Lab # Demo (A) Starting Substances - Demo (B) Physical Properties of starting substances Changes Observed After Mixing (e.g., state, colour change, gas formation etc.) - - - - 1 - - 2 - - - - 3 4 5 - Physical or Chemical Change? Results of Gas Test or NA (not applicable) Group Members: