Charles' Law Notes
advertisement

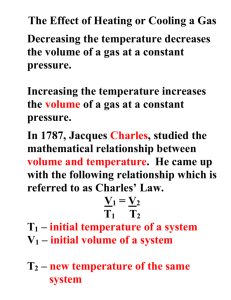
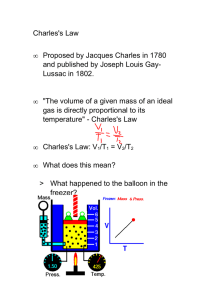
Charles’ Law Notes Charles’ Law discovered by French physicist, Jacques Charles in 1787 first person to fill balloon with _____________________________and make solo balloon flight Temperature vs. Volume If P is constant, gases ____________________________ when heated. When T______________________, gas molecules move ______________________and collide with the walls more often and with greater force. To keep the P___________________, the V must _______________________. Charles’ Law: T and V Charles’ Law states that ___________________________________________________________________ ______________________________________________________________________________________________ V = kT or k = V/T k is a constant for a certain sample of gas that depends on the mass of gas and its pressure. What kind of graph is V vs. T? (Draw graph in space below) If we have a set of new conditions for the same sample of gas, they will have same k so: lV1/T1 = k = V2/ T2 lV1/T1 = V2/ T2 PROBLEM… If we use the Celsius temperature system, we could end up with negative values when using Charles’ gas law. SOLUTION… To use Charles’ Law we need a temperature scale that has no negative values; this is the ________________________________________________________________ starts at -273.15 ° C = absolute zero = 0 Kelvin (K) The lowest possible temperature for this system is ___________________. Example: Charles’ Law A gas with a volume of 600 mL has a temperature of 30 0C. At constant pressure the gas is heated until the gas expands to 1,200 mL. What is the new temperature of the gas if the pressure remains constant? SOLUTION 1.The first step is to convert the temperature from Celsius to Kelvin: 2.Rearrange Charles’ law to solve for the new temperature: