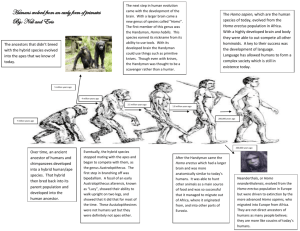
possibility of African apes being descendants of
advertisement

MORPHOLOGICAL DISTANCE BETWEEN AUSTRALOPITHECINE, HUMAN AND APE SKULLS Human Evolution 11: 35-41, 1996 This paper attempts to quantify the morphological difference between fossil and living species of hominoids. The comparison is based upon a balanced list of craniodental characters corrected for size (Wood & Chamberlain, 1986). The conclusions are: craniodentally the australopithecine species are a unique and rather uniform group, much nearer to the great apes than to humans; overall, their skull and dentition do not resemble the human more than the chimpanzee’s do. Key words: human evolution, hominids, apes, skull, Australopithecus, Homo erectus, chimpanzee, gorilla Introduction The australopithecine species are commonly considered to be “hominids” beeause they lack some of the features that characterize the living apes, and display certain humanlike characters. Yet it has often been argued that their humanlike characters might be primitive and indeed many of these characters are found in premature African apes - and that the australopiths should not be included in the evolutionary branch towards humans, but instead are a unique group of apes or might even be closer phylogenetically to the African apes than to humans (e.g., Kleindienst, 1975; Goodman, 1982; Gribbin & Cherfas, 1983; Oxnard, 1984; Hasegawa et al., 1985; Edelstein, 1987; Verhaegen, 1990; 1994). The aim of this paper is to objectivate morphological resemblances of australopithecine species with living hominoid species. (To establish phylogenetic relationships, biomolecular comparisons of nucleic acids or proteins are preferable to morphological comparisons, but it does not seem very probable that extraction of enough DNA or protein from fossil bone will ever become possible.) Methods I have used the comparative data of Wood and Chamberlain (1986) because: Their data are likely to be comparable since they stem from the same source. They use a balanced list of 39 characters, i.e., "selected to provide a relatively comprehensive coverage of the head" without much functional or morphological overlapping. The 39 characters stem from: cranial vault and endocranium (11 V), face (7 F), palate and maxilla plus dentition (7 P), cranial base (5 B), and mandible plus dentition (9 M). Wood and Chamberlain do not use the "raw" metrical data, but ratios, which "help to reduce, if not actually eliminate, differences due to absolute size". Postcranial data (more scarce and difficult to attribute to a certain species) are not included in their list. Since the data for the 39 characters were not available for all species, I selected two (overlapping) Character Groups (only characters V9 and B5 were not used at all): I. one of 32 characters (82 %) that were available for 8 species: Hylobates, Pongo, Pan troglodytes, Gorilla, A. africanus, A. boisei, H. erectus and H. sapiens (characters V1-8,1011, F1-7, P1-3,5-6, B1,4, M-6,8-9); II. one of 27 characters (69 %) that were available for 7 species: Pongo, Gorilla, Pan troglodytes, Homo sapiens, Australopithecus africanus, A. robustus and A. boisei (characters V1,7-8, F1-7, P1-7, B1-3, M1,4-9). This second Group was added since it included data from a third australopithecine species, A. robustus. The data for Pan paniscus, A. afarensis and H. neanderthalensis could not be used, since they were available for too few characters. The data for “H. habilis” (which included ER-1470, ER-1813, and OH material) were not used since they might belong to more than one species. A simple method measured the relative overall craniodental distance between the different (fossil as well as living) hominoid species without considering any of these species as an outgroup a priori: Each character had to have equal weight. For each species and each character, the sum of the differences with the same character in the other species was given an arbitrary weight of 1000, i.e., each of the differences with the other species was divided by the sum of these differences and multiplied by 1000. Tables Ia and IIa show the mean results of all (32 or 27) characters for all (8 or 7) species. These results, of course, are not directly proportional to the morphological distance, but indicate that the difference between species A and B is larger or smaller than that between A and C. As an example, Figure 1 shows the calculation of the results for Character Group II (and more in particular for A. boisei). These results in Tables Ia and IIa for each species were made more comparable with those for the other species in the same Character Group (e.g., for interpreting the diagrams, see below) by multiplying them by a correction factor consisting of the sum (/1000) of the differences of the other species with that species (see Figure l). This yielded Tables Ib and IIb. (This correction exaggerates the results of the most aberrant species (e.g., H. sapiens in Table II), but does not change the order of differences.) For illustrating which one of the living species resembled a fossil species most, the diagrams of Figure 2 were constructed. Since all results are relative, the diagrams could be made clearer by equalling one of the species to zero. In this case, Pongo, which was nearest to the mean species, was taken as the reference (this choice, of course, does not influence the conclusions): in Tables Ib and IIb, the results comparing Hylobates, Gorilla, P. troglodytes and H. sapiens with the fossils were subtracted from the results of Pongo, so that a positive result (above the x-axis) means that the fossil resembles the living species more than it resembles Pongo craniodentally; a negative (below the x-axis), less. (For comparing the diagrams between both Character Groups, the results for Group I could have been multiplied by 5198/3954, which is the quotient of the sums of the differences within Group II and I using only the species common to both Groups (i.e., omitting the results for H. erectus, A. robustus and Hylobates). This second correction factor (even less than the first) would not have influenced the conclusions.) Discussion The tables show that morphologically the great hominoids form three clusters: Homo, the australopithecines, and the great apes. 1) The human skull is unique and differs from that of the great apes even more than the gibbon does. Homo is about equidistant from australopithecines and chimpanzees (though evolutionarily he is probably closer to A. africanus than to Pan, only because A. africanus lived almost three million years nearer to the common ancestor). H. erectus in Group I seems to be on the way to H. sapiens. He is about equidistant from H. sapiens, P. troglodytes and A. africanus, but differs from the australopithecines even slightly more than Pongo does. 2) The australopithecine skulls resemble each other more than they resemble the apes (even the African apes) and certainly humans; A. robustus stands somewhere between A. africanus and A. boisei, but nearer to A. boisei. In comparison with the living species (Figure 2): A. africanus in both Character Groups is closest to the chimp, and closer to chimpanzees, gorillas and orangutans than to humans (and to gibbons in Group I); A. robustus in Group II also is morphologically closest to the chimpanzee, and much closer to chimp, gorilla and orang than to humans (gibbons were not included in this comparison); A. boisei in both Groups is closer to G. gorilla than to Pan troglodytes (in contrast with the South African fossils), and very different from Homo, somewhat more different than A. africanus is from Homo. A. boisei (who lived later) more than A. africanus (who lived earlier) resembles the living African apes compared with humans or orangs or gibbons (Figure 2). In Diagram II of Figure 2, A. robustus also resembles the African apes more than A. africanus does in comparison with humans. This indicates that the australopithecines (from graciles to robusts) were evolving in the African ape direction - whether in parallel with the apes (see Ferguson, 1989) or not (Verhaegen, 1994). 3) The great apes (even including Pongo) resemble each other even more than H. erectus and H. sapiens in Group I resemble each other, in spite of the evolutionary distance between the apes (cf. the African apes and Pongo split perhaps ten times earlier than H. erectus and H. sapiens). They resemble each other more than A. boisei resembles A. africanus. This points to a remarkable degree of conservatism and/or of parallelism in cranial evolution of these three great ape species (and to a remarkably fast evolution of Pleistocene Homo). Yet, chimps, somewhat more than gorillas, resemble Homo more than orangs and certainly gibbons do (in accordance with the biochemical resemblances). All this implies that the craniodental evidence provides no ground for the anthropological custom of using the living African hominoids as an outgroup when comparing australopithecines with humans or when reconstructing hominoid phylogenetic trees: if the australopithecine species are considered to be hominid, the great apes and certainly the African apes should also be called hominid, since they resemble the australopiths more than humans do, and they do not differ from humans more than the australopiths do (Figure 3). The australopithecines are often assumed to be hominids on the basis of their postcranial features (not included in Wood and Chamberlain’s list), but many authors argue that locomotorically australopithecines differed more from humans than from the African apes (for discussion and references, see especially Oxnard, 1984; and Verhaegen, 1990, 1993, 1994). In this respect too, the australopithecines could have had unique adaptations (Oxnard, 1984) for an environment or lifestyle that no longer exist. (For instance, there is dental as well as paleo-environmental evidence that the later australopiths fed partly on bamboo or reed or papyrus (Du Brul, 1977; F. E. Grine, pers. comm.; Puech, 1992, and pers. comm.; Verhaegen, 1992), possibly wading bipedally in the shallow waters where most fossils are discovered (discussion in Verhaegen, 1993).) Although Gorilla and Pan skulls resemble each other morphologically (Tables Ib and IIb), both species differ biochemically (in DNA and proteins) even more than Homo and Pan (e.g., Horai et al., 1995). Since synchronous parallel evolution in related species in response to a climatic change appears to be the rule (e.g., White and Harris, 1977; Seger, 1987, Gibbs and Grant, 1987; Bown et al., 1994; theoretical considerations in Silson, 1988), some African ape features that are usually assumed to be primitive might instead have developed in parallel in gorillas and in chimpanzees. The possibility should even be considered that, if australopiths are more closely related to the African apes than to humans (be it, of course, on morphological grounds, see Figure 3), some australopithecines might evolutionarily be closer to chimpanzees and others to gorillas (discussion in Verhaegen, 1994). Conclusions This comparison of 37 craniodental characters of fossil and living apes and humans yields no indication that any of the australopithecine species has evolved in the human direction. South African australopithecine skulls are morphologically closest to the chimpanzee among the living hominoids, and A. boisei is closest to the gorilla among the living hominoids. Human craniodental evolution appears to have been very fast the last one or two million years. These conclusions could be verified and extended when more (including postcranial) data on living (e.g., P. paniscus) and fossil hominoids (adult and premature) will become available. Tables Craniodental differences between hominoid species. Tables Ia and Ib based on 32 characters (8 species). Tables IIa and IIb based on 27 characters (7 species). Tables Ib and IIb corrected (see text and Figure 1). References Bown T. M., Holroyd P. A. and Rose K.D., 1994. Mammal extinctions, body size, and paleotemperature. Proceedings of the National Academy of Sciences USA, 91: 104036. Du Brul, E. L., 1977. Early hominid feeding mechanisms. American Journal of Physical Anthropology, 47: 305-320. Edelstein S. J., 1987. An alternative paradigm for hominoid evolution. Human Evolution, 2: 169-174. Ferguson W.W., 1989. A new species of the genus Australopithecus Primates-Hominidae from the Plio/Pleistocene deposits West of Lake Turkana in Kenya. Primates, 30: 223232. Gibbs, H. L. and Grant P. R., 1987. Oscillating selection on Darwin's finches. Nature, 327: 511-513. Goodman M., 1982. Biomolecular evidence on human origins from the standpoint of Darwinian theory. Human Biology, 54: 247-264. Gribbin J. and Cherfas J., 1983. The Monkey Puzzle. London: Triad. Hasegawa M., Kishino H. and Yano T., 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 2: 160-174. Horai S., Hayasaka K., Kondo R., Tsugane K. and Takahata N., 1995. Recent African origin of modern humans revealed by complete sequences of hominoid mitochondrial DNAS. Proceedings of the National Academy of Sciences USA, 92: 532-536. Kleindienst M.R., 1975. On new perspectives on ape and human evolution. Current Anthropology, 16: 644- 646. Oxnard C. E., 1984. The Order of Man. New Haven: Yale University Press. Puech P.-F., 1992. Microwear studies of early African hominid teeth. Scanning Microscopy, 6: 1083-1088. Seger J., 1987. El Nino and Darwin's finches. Nature, 327: 461. Silson R.G., 1988. Additive Genes in Evolution and Selection. Tring: Greenfield Publications. Verhaegen M., 1990. African ape ancestry. Human Evolution, 5: 295-297. Verhaegen M., 1992. Did robust australopithecines partly feed on hard parts of Gramineae? Human Evolution, 7: 63-64. Verhaegen M., 1993. Aquatic versus savanna: comparative and paleo-environmental evidence. Nutrition and Health, 9: 165-191. Verhaegen M., 1994. Australopithecines: ancestors of the African apes? Human Evolution, 9: 121-139. White T. D. and Harris J. M., 1977. Suid evolution and correlation of African hominid localities. Science, 198: 13-21. Wood B. A. and Chamberlain A.T., 1986. Australopithecus: grade or clade? In (B. A. Wood, L. Martin and P. Andrews, eds). Major Topics in Primate and Human Evolution, pp. 220-248, Cambridge University Press, Cambridge. AUSTRALOPITHECINES: ANCESTORS OF THE AFRICAN APES? Human Evolution 9: 121-139, 1994 Since australopithecines display humanlike traits such as short ilia, relatively small front teeth and thick molar enamel, they are usually assumed to be related to Homo rather than to Pan or Gorilla. However, this assumption is not supported by many other of their features. This paper briefly surveys the literature concerning craniodental comparisons of australopith species with those of bonobos, common chimps, humans and gorillas, adult and immature. It will be argued, albeit on fragmentary data, that the large australopiths of East Africa were in many instances anatomically and therefore possibly also evolutionarily nearer to Gorilla than to Pan or Homo, and the South African australopiths nearer to Pan and Homo than to Gorilla. An example of a possible evolutionary tree is provided. It is suggested that the evidence concerning the relation of the different australopithecines with humans, chimpanzees and gorillas should be re-evaluated. Key words: Hominid evolution, Australopithecus, robust polyphyly, gorilla, chimpanzee, bonobo, Lucy, Taung, molecular clock. Introduction Biomolecular data place the Homo/Pan splitting time between 8 and 4 Myr BP (e.g. Sarich, 1977; Hasegawa et al., 1987, 1988, 1989; Caccone & Powell, 1989). This means that at the time of the earliest undoubted australopithecines (ca. 4 Myr BP), the differences between human and chimpanzee ancestors were much less than those between present-day Homo and Pan, so that it is difficult to decide whether a particular fossil of that age belonged to the Homo or to the Pan clade. No a priori reason exists therefore to reject the idea that the African apes may have had australopith ancestors. Several people had contemplated this possibility even before Sarich & Wilson (1967) initiated the drastic reduction of the estimated Homo/Pan splitting time (e.g. Woodward, 1925; Smith, 1925; Keith, 1925a, b; 1931, p.115; Schultz, 1941, p.100; A. Hrdlicka in Howells, 1985; W. Abel; W. L. Straus, S. Zuckerman, E. H. Ashton in Reader, 1988, p. 89 and p. 124). Following the introduction of the biomolecular evidence, the idea has revived (e.g. Kleindienst, 1975; Goodman, 1982; Gribbin & Cherfas, 1983; Hasegawa et al., 1985; Edelstein, 1987; Verhaegen, 1990; Trevino, 1991, p. 14-15). This approach could also explain the discrepancy between the enormous number of fossil finds and the apparent total absence of fossil African ape ancestors from a period covering at least the last four million years (Gribbin & Cherfas, 1983; Verhaegen, 1990). The usual explanations offered are that paleontologists have not worked in the appropriate areas, or that the probability of fossilization in the tropical forests, where the ancestral apes presumably lived, is very low because of the relative acidity or the wetness of the soil (e.g. G. S. Krantz in Kleindienst, 1975). These explanations are hard to reconcile with the numerous discoveries of forest-dwelling bovids, suids, monkeys, dryopithecines and probable early relatives of the orang-utan (Kleindienst, 1975; Kortlandt, 1975; cf. Pilbeam, 1982; Andrews & Cronin, 1982). When Dart (1925) discovered the skull of Taung, he believed that it was in the human lineage because it showed what he called “humanoid” features such as relatively small canines and forward situation of the foramen magnum. His proposal was promptly rejected by nearly all his colleagues (e.g. Keith, 1925a,b; Smith, 1925; Woodward, 1925; Duckworth, 1925), who saw in Taung nothing more than a sort of young chimp or perhaps gorilla. They were supported in their opinion by the Piltdown skull, which showed a rather ape-like dentition together with a big brain, almost the opposite of the Taung child. But later, when Kenneth Oakley unmasked Piltdown Man as a fraud and Robert Broom concluded from his studies of postcrania that the South African australopithecines were bipedal, opinions about Taung changed and the australopiths became accepted as being closer to man than to apes. This paper argues that the nearly general acceptance around 1950 of W. E. Le Gros Clark’s ideas, following Dart and Broom, that the South African australopiths were closer to humans than to “pongids” (mostly based on comparisons of their pelvis and dentition, often with male gorillas, e.g. Le Gros Clark, 1978, first edition 1955) might have been an overreaction after the unmasking of Piltdown, and that the anthropologists’ first impressions that Taung was a fossil species of Pan - should be reconsidered. (That Taung was closer to Homo than to Gorilla and certainly Pongo, is of course not contested in this paper). Homo-like features in australopiths: primitive? In imitation of Dart, Broom and Le Gros Clark, the australopithecine species are now usually considered to be closer relatives of humans than of apes. This opinion is based especially on their locomotor and dental features. Ventral position of foramen magnum It is generally accepted that the australopiths were more bipedal than present-day gorillas and common chimps, mostly because of the Laetoli footprints almost 4 Myr BP, the short ilia of Lucy and Sts.14, the broader calcaneus and the more human-like orientations (though rather ape-like anatomy) of the ankle and knee articulations of the Hadar specimens (Stern & Susman, 1983; Latimer et al., 1987), and the more ventral position of the foramen magnum in many australopiths. “Early australopithecines are linked with living humans on the basis of shared characters related to bipedalism” (Andrews, 1992), but it is often argued that the African apes’ ancestors also were more bipedal (theory of W. L. Straus; see Coon 1954; Kleindienst, 1975; Goodman, 1982; Gribbin & Cherfas, 1983; Hasegawa et al., 1985; and esp. Edelstein, 1987; cf. Schultz 1949, p. 205). Indeed, that the African apes could evolve from digiti-palmigrades (all other primates, including human infants) to knuckle-walkers implies that they went through a phase where the arms were barely used for pronograde locomotion (cf. Edelstein, 1987); an intermediate phase of orthograde arm-hanging or brachiation insufficiently explains knuckle-walking since neither orangutans nor hylobatids show traces of knuckle-walking. Also, most anthropoids (especially the young) occasionally walk on two legs, and bipedal tendencies are very striking in the African apes (but virtually absent in Pongo). Chimpanzee fetuses shortly before birth show humanlike feet with ventrally oriented and adducted first digital rays (Coon, 1954). Common chimps often walk bipedally on muddy terrain (Nishida, 1980), and bonobos are even more frequently bipedal (Zihlman et al., 1978; De Waal, 1988). “When they are on the ground, anthropoid apes... often walk erect, and the mountain gorilla's foot, indeed, is already similar to man’s” (Rensch, 1972, p. 63; see also p. 130; Schultz, 1950; Edelstein, 1987). In addition, of all primates, only the African hominoids are fully plantigrade (Gebo, 1992): “Orangutans have further enhanced foot mobility by adapting their feet for suspension and thus similarly utilize foot positions where the heel does not touch the substrate. Chimpanzees and gorillas represent an alternative pattern (plantigrady), in which the heel contacts the surface of the support at the end of the swing phase, especially during terrestrial locomotion. Thus chimpanzees and gorillas possess feet adapted for both arboreal and terrestrial substrates. African apes also share several osteological features related to plantigrady and terrestrial locomotion with early hominids. Humans and African apes are very similar in their use of plantigrady when moving or standing upon a terrestrial substrate and this pattern of foot use is extremely different from what characterizes all other primates”. Also, young gorillas and chimpanzees have foramina magna more ventral than adults and well within the range of A. africanus Sts.5 (e.g. Ashton & Zuckerman, 1952; Schultz, 1955). Even in adults, the foramen has the same position indices in gracile (Sts.5) and robust australopithecines (KNM-ER 406) as in bonobos (Kimbel et al., 1984, table 9). Masters et al. (1991): “Since Taung was perhaps 3.5 years of age at death (Bromage and Dean, 1985), the position of the foramen magnum may not have achieved its adult status. This contradicts the assessment made by Dart (1925), who interpreted the position of the foramen magnum as indicating bipedal locomotion in Australopithecus africanus”. Thick enamel Thicker molar enamel was formerly treated as a reliable sign of affinity with the human line, so that species such as “Ramapithecus” (now included in Sivapithecus and usually considered to have Pongo as its closest living relative, see Pilbeam, 1982; Andrews & Cronin, 1982) were designated as possible human ancestors on the strength of this evidence. But this is no argument for a closer affinity of the australopithecines with humans than with African apes, since Martin (1985) argues that the extant great apes have secondarily reduced enamel - slight in the case of Pongo and more marked in Gorilla and Pan (but see also Beynon et al., 1991). It is in no way inconsistent with australopiths being ancestral to African apes (Martin, 1985): “thick pattern 3 enamel does not identify a hominid. Moreover, the common ancestor of the great apes and man, and of the African apes and man, would have had teeth resembling those of hominids... Of the living members of the great ape and human clade, only Homo sapiens retains the condition of enamel thickness and development from the common ancestor of the clade and can therefore be regarded as the most dentally primitive member of it”. Smaller anterior dentition The pronounced prognathism and large incisors and very large canines of the adult males of G. gorilla and P. troglodytes are thought to exclude australopith ancestors, since most robust australopiths had “flat” faces and (at least in comparison with their enormous back teeth) small anterior teeth. But this evidence is not conclusive: (1) A. afarensis and A. africanus also possessed moderately projecting canines, and the robust australopiths could have been extinct side-branches specialized for extremely tough food (e.g. Verhaegen, 1992), (2) even in robust australopiths (SK 23, Natron, L.7-125), the indices of the basic rectangle of the mandible are within the range of these of common chimps but outside those of humans (Kinzey, 1970); (3) in many robust specimens, the front teeth are so much worn that it is difficult to estimate how long their unworn canines would have been (but at least the A. boisei from Chesowanja showed unworn canines which were rather short), (4) in Gorilla and Pan “with advancing age, canines tend to wear flat to the level of the incisors” (Ryan & Johanson. 1989); (5) bonobos “have relatively small and only slightly dimorphic canine teeth” (Zihlman et al., 1978); (6) “infant great apes have flat or orthognathic faces like modern humans” (Aiello & Dean, 1990, p, 197); (7) some specimens of H. erectus had maxillary diastemata of 6 mm, as large as an orang’s (Howells, 1959, p. 157; Rensch, 1972, p. 36), and much larger than in the robust australopiths (who lived earlier); (8) selection for larger or smaller teeth can theoretically occur in very short evolutionary periods (cf. Silson, 1988, p. 19), and is claimed to have been demonstrated in only a few thousand years in human populations (Calcagno & Gibson, 1988); (9) the marked difference in prognathism between Negroes and Whites (e.g. Howells, 1959, p.269; Kinzey, 1970, fig. IA-B) developed in a time span of only about 200,000 years (Cann et al., 1987; Vigilant et al., 1991). Moreover, it is not very likely that the primitive African hominoid condition included ape-like pronounced prognathism and very long canine teeth. See, for instance, Kinzey’s (1970) comparisons of the basic rectangle of the mandible in different Haplorhini (in Kenyapithecus africanus, e.g., it resembles Homo rather than Pan), or the anatomy of the face in infant chimpanzees (orthognathism with relatively short milk canines and vertical mandibular symphysis). Australopiths resemble young Pan or Gorilla At first sight, australopith skulls are more reminiscent of African apes, especially juveniles and subadults, than of humans (even Le Gros Clark calls them ape-like creatures): the general morphology of the gracile crania resembles that of bonobos or common chimps, and the larger crania are more gorilla-like (e.g. Lewin, 1987, p. 260; Zihlman et al., 1978; Rensch, 1972, p. 40; Robinson, 1960; Kennedy, 1991, fig.1). This is not contradicted by a more detailed look at their anatomy. Table 1 gives a few striking quotations about ape-like features of australopith skulls in general; Table 2 provides quotations about gorilla-like cranial features in large East African fossils; Table 3, about chimpanzee-like features in africanus and robustus crania. It is a pity that this paper has to rely so heavily upon the anthropologists’ impressions (quoted in Tables 1, 2 and 3), but extensive comparisons with enough species (including several extinct hominid species, African hominoids and humans) are rather scarce. Even in recent excellent and thorough textbooks (e.g. Conroy, 1990; Aiello & Dean, 1990), australopith fossils are often compared only with man and one of the great apes (usually the common chimp), and detailed comparisons of the australopithecine features with humans and all three African ape species (not to mention orang-utans), preferably of different subspecies (e.g. high- vs lowland gorilla), ages and sexes, are surprisingly rare in the literature (but see e.g. Schultz, 1955; McHenry, 1983; Demes, 1988). Because of these anthropocentric viewpoints, the differences between the African ape species - like those between the different australopith species - are often underestimated. In fact, Pan is more closely related to Homo than to Gorilla biochemically (see below), and Groves & Paterson (1991) in their computer analysis of 89 anatomical features, conclude that Pan is even morphologically slightly nearer to Homo than to Gorilla. Table 1 - Some quotations on ape-like features in australopith crania “The evolution of the australopithecine crania was the antithesis of the Homo line. Instead of becoming less ape-like, as in Homo, they become more ‘ape-like’. Cranial proportions and ectocranial features that were thought to be unique among pongids evolved separately [? M. V.] in the australopithecines parallel [? M. V.] with the great apes. The features of KNM-WT 17000, therefore, are not as ‘primitive’ as they look. The robust Australopithecus did not evolve from a big-toothed pongid ancestor with large cranial superstructures, but from a small-toothed hominid with a rounder, smoother ectocranium, like A. africanus”. Ferguson, 1989b. “Plio-Pleistocene hominids had markedly abbreviated [enamel] growth periods relative to modern man, similar to those of the modem great apes”. Bromage & Dean, 1985. “Enamel thickness has been secondarily reduced in the African apes and also, although at a different rare and extent, in the orang-utan. Thick enamel, previously the most important characteristic in arguments about the earliest hominid, does not therefore identify a hominid”. Martin, 1985 (but Beynon et al., 1991). In the South African fossils including Taung, “sulcal patterns of seven australopithecine encocasts appear to be ape-like rather than human-like”. Falk, 1987. “Cranial capacity, the relationship between endocast and skull, sulcal pattern, brain shape and cranial venous sinuses, all of these features appear to be consistent with an ape-like external cortical morphology in Hadar early hominids”. Falk, 1985. In the type specimen of A. afarensis, “the lower third premolar of ‘A. africanus afarensis’ LH-4 is completely apelike”. Ferguson, 1987b. “A. afarensis is much more similar cranially to the modern African apes than to modern humans”. Schoenemann, 1989. “Olson's assertion that the lateral inflation of the A.L. 333-45 mastoids is greater than in any extant ape is incorrect if the fossil is compared to P. troglodytes males or some Gorilla mates and females. Moreover, the pattern of pneumatization in A. afarensis is also found only in the extant apes among other hominoids”. Kimbel et al., 1984. “Prior to the identification of A. afarensis the asterionic notch was thought to characterize only the apes among hominoids. Kimbel and Rak relate this asterionic sutural figuration to the pattern of cranial cresting and temporal bone pneumatization shared by A. afarensis and the extant apes”. Kimbel et al., 1984. “... the fact that two presumed Paranthropus [robustus] skulls were furnished with high sagittal crests implied that they had also possessed powerful occipital crests and ape-like planum nuchale... Nuchal crests which are no more prominent - and indeed some less prominent - will be found in many adult apes”. Zuckerman, 1954b. In Sts.5, MLD-37/38, SK-47, SK-48, SK-83, Taung, KNM-ER 406, O.H.24 and O.H.5, “craniometric analysis showed that they had marked similarities to those of extant pongids. These basicranial similarities between Plio-Pleistocene hominids and extant apes suggest that the upper respiratory systems of these groups were also alike in appearance... Markedly flexed basicrania [are] found only in modern humans after the second year...”. Laitman & Heimbuch, 1982. “The total morphological pattern with regard to the nasal region of Australopithecus can be characterized by a flat, non-protruding nasal skeleton which does not differ qualitatively from the extant nonhuman hominoid pattern, one which is in marked contrast to the protruding nasal skeleton of modern H. sapiens”. Franciscus & Trinkaus, 1988. Table 2 - Quotations on gorilla-like features in large East African australopith crania “Incisal dental microwear in A. afarensis is most similar to that observed in Gorilla”. Ryan & Johanson, 1989. The composite skull reconstructed mostly from A.L.333 specimens “looked very much like a small female gorilla”. Johanson & Edey, 1981, p. 351. “Other primitive [or advanced gorilla-like? M. V.] features found in KNM-WT 17000, but not know or much discussed for A. afarensis, are: very small cranial capacity; low posterior profile of the calvaria; nasals extended far above the frontomaxillar suture and well onto an uninflated glabella; and extremely convex inferolateral margins of the orbits such as found in some gorillas”. Walker et al., 1986. As for the maximum parietal breadth and the biauriculare in O.H.5 and KNM-ER 406 “the robust australopithecines have values near the Gorilla mean: both the pongids and the robust australopithecines have highly pneumatized bases”. Kennedy, 1991 (see also his fig. 1). In O.H.5, “the curious and characteristic features of the Paranthropus skull... parallel some of those of the gorilla”. Robinson, 1960. The A. boisei “lineage has been characterized by sexual dimorphism of the degree seen in modern Gorilla for the length of its known history”. Leakey & Walker, 1988. A. boisei teeth showed “a relative absence of prism decussation”; among extant hominoids, “Gorilla enamel showed relatively little decussation ...”. Beynon & Wood, 1986 (cf. Beynon et al., 1991). Table 3 - Quotations on chimp-like features in South African australopith crania “Alan [Walker] has analysed a number of Australopithecus robustus teeth and they fall into the fruit-eating category. More precisely, their teeth patterns look like those of chimpanzees... Then, when be looked at some Homo erectus teeth, be found that the pattern changed”. Leakey, 1981, pp. 74-75. “The ‘keystone’ nasal bone arrangement suggested as a derived diagnostic of Paranthropus [robustus] is found in an appreciable number of pongids, particularly clearly in some chimpanzees”. Eckhardt, 1987. “P. paniscus provides a suitable comparison for Australopithecus [Sts.5]; they are similar in body size, postcranial dimensions and... even in cranial and facial features”. Zihlman et al., 1978. “A. africanus Sts.5, which... falls well within the range of Pan troglodytes, is markedly prognathous or hyperprognathous”". Ferguson, 1989a. In Taung, “I see nothing in the orbits, nasal bones, and canine teeth definitely nearer to the human condition than the corresponding parts of the skull of a modern young chimpanzee”. Woodward, 1925. “The Taung juvenile seems to resemble a young chimpanzee more closely than it resembles L338y-6”, a juvenile A. boisei. Rak & Howell, 1978. “In addition to similarities in facial remodeling it appears that Taung and Australopithecus in general, had maturation periods similar to those of the extant chimpanzee”. Bromage, 1985. “I estimate an adult capacity for Taung ranging from 404-420 cm2, with a mean of 412 cm2. Application of Passingham’s curve for brain development in Pan is preferable to that for humans because (a) brain size of early hominids approximates that of chimpanzees, and (b) the curves for brain volume relative to body weight are essentially parallel in pongids and australopithecines, leading Hofman to conclude that ‘as with pongids, the australopithecines probably differed only in size, not in design’”. Falk, 1987. In Taung, “pneumatization has also extended into the zygoma and hard palate. This is intriguing because an intrapalatal extension of the maxillary sinus has only been reported in chimpanzees and robust australopithecines among higher primates”. Bromage & Dean, 1985. “That the fossil ape Australopithecus [Taung] ‘is distinguished from all living apes by the... unfused nasal bones…’ as claimed by Dart (1940), cannot be maintained in view of the very considerable number of cases of separate nasal bones among orang-utans and chimpanzees of ages corresponding to that of Australopithecus”. Schultz, 1941. Only a few possibly relevant data linking an australopith fossil with one of the extant African hominoids could be obtained from the literature (cf. Tables 2 and 3). Uniquely derived cranial features of A. boisei and Gorilla concern: some incisal microwear features (Ryan & Johanson, 1989; though acquired ontogenetically, tooth wear reflects phylogenetic adaptations); enamel prism decussation (Beynon & Wood, 1986; cf. Beynon et al., 1991); orbital morphology (KNM-WT 17000, see Walker et al., 1986); body size (but see also McHenry, 1991). Uniquely derived features of South African australopiths with Pan and Homo concern: mandibular premolar root morphology (Wood et al., 1988; see also below). Uniquely derived features of A. robustus and Pan concern: tooth microwear (e.g. Leakey, 1981, p.74); nasal bone arrangement (Eckhardt, 1987); maxillary sinus topology (Bromage & Dean, 1985; see also Cave & Wheeler Haines, 1940). Not obtained were: uniquely derived features of South African fossils with Gorilla; of A. boisei with Pan; and of any australopith with Homo (i.e. features that are absent from all African ape species, mature and immature). Jenkins (1991): “Tobias (1988) prepared a comparative list of the cranial, mandibular, dental and endocranial traits for H. habilis, A. africanus, A. robustus, and A. boisei to determine evidence for cladogenetic relationships. His tabular summaries enumerate numerous shared derived characters of all four taxa. However, he did not include any outgroup comparisons. In this poster, data for two outgroups [? M. V.], composed of Gorilla gorilla and Pan troglodytes, were compiled and compared to Tobias’ evaluations of H. habilis, A. africanus and A. boisei. The results show that numerous traits he used are also shared with Gorilla and Pan...” Thick molar enamel and small anterior dentition are discussed above. Orthognathism, intermediate position of foramen magnum, relatively “short” arms, lateral plantar process of calcaneus, longer and adducted first metatarsals, etc. are seen in bonobos or/and immature apes. Lucy’s short ilium is not a good case: overall, her pelvis is as distinct from the human as it is from the chimpanzee’s (e.g. Stern & Susman 1983), and the Sterkfontein Sts.14 pelvis (notably the ischium) is even more chimp-like (Broom & Robinson, 1950; Oxnard, 1984, fig. 10.1); short ilia (in proportion to trunk length) as in monkeys and humans are probably the ancestral condition, so that Coon (1954) could assert that, in pelvic morphology, apes look less like monkeys than humans do (cf. Schultz, 1950, fig. 6). W. L. Straus (in Schultz, 1936, p. 431): “The human ilium would seem most easily derived from some primitive member of a preanthropoid group, a form which was lacking many of the specializations, such as reduction of the iliac tuberosity and anteacetabular spine and modification of the articular surface, exhibited by the modern apes. I wish to emphasize here that the anthropoid-ape type of ilium is in no sense intermediate between the human and lower mammalian forms. Its peculiar specializations are quite as definite as those exhibited by man, so that it appears very unlikely that a true anthropoid-ape form of ilium could have been ancestral to the human type”. Overall, the more human-like features of the australopith hindlimbs are less abundant than the more ape-like features (summarized in Oxnard, 1984, Nota Bene following p. 334; and in Verhaegen, 1990). Moreover, it has been argued that all these human-like features (e.g. the superhumanly broad sacrum, long femoral neck and valgus knee) could have been correlated with some sort of bipedalism in the ancestral African hominoids (see the discussion above). With the apparent exception of the front teeth reduction and the relative orthognathism in A. boisei and A. robustus (but juvenile African apes also are orthognathic, see Schmid & Stratil, 1986; Aiello & Dean, 1990, p. 197), later large australopith skulls (KNM-WT 17000) show more gorilla features than earlier ones (from A.L.333), and later smaller ones show more chimpanzee features than earlier ones (Taung more than Sts.5, and much more than Lucy). See, for instance, the first quotation of Table l; for KNM-WT 17000, Table 2; for Taung, Table 3, and Falk et al. (1989); and for Lucy, Ferguson (1987b). (The same could be true of the postcrania: see Verhaegen (1990) and the discussion of the distal humerus below. Nevertheless, most Kromdraai and Swartkrans remains are usually described as being intermediate between humans and chimps but more human- than ape-like (especially the lower limb features, e.g. the adducted hallux), and more human-like than those of Hadar (Susman, 1989; Gebo, 1992). This does not necessarily contradict the evolutionary trees proposed in this paper: (1) although most Swartkrans fossils certainly belong to A. robustus, a few probably represent Homo (Susman, 1989); (2) the earliest split is not that between humans and (African) apes, but that between Pan-Homo and Gorilla (see below), and A. robustus undoubtedly belonged to Pan-Homo rather than to Gorilla; (3) at the time of A. robustus there already existed much more humanlike fossils (e.g. KNM ER-1470 and 148l), so that A. robustus must have belonged either to Pan or to an extinct side-branch of Pan-Homo; (4) prenatal apes show adducted great toes (see above), and Pan (notably paniscus) is more bipedal than Gorilla).) In spite of the scarcity of comparative data from single sources, a few figures regarding skulls and dentitions (the postcrania are briefly discussed in Verhaegen, 1990) are brought together in Table 4a (comparative measurements from different sources were not used), and some preliminary conclusions emerge from it (Table 4b): (1) The figures of the large afarensis skulls from A.L.333 are rather ape-like, with more bonobo-like foramen magnum indices, chimp-like frontal bone, and rather gorilla-like dental features. (2) Overall, A. africanus from Makapansgat and Sterkfontein resemble Pan rather than Gorilla or Homo, and in bite force and foramen magnum indices, bonobos rather than common chimps. (3) The figures of A. robustus from Swartkrans, mostly regarding the dentition, are generally intermediate between those of common chimp and gorilla. (4) A. boisei KNM-ER 406 and O.H.5, in spite of the differences between them, are more gorilla-like (KNM-ER 406 is even super-gorilla in bite force). Every australopith species in this Table thus appears morphologically nearer to at least one of the African ape species than it is to humans. Robust polyphyly? Biomolecular results leave no doubt that Pan is genetically closer to Homo than to Gorilla (e.g. Goodman, 1982; Hasegawa et al., 1985, 1987, 1988; Caccone & Powell, 1989; Sibley et al., 1990; Gonzalez et al., 1990; Ruvolo et al., 1991; Begun, 1992), and contrary to the prevailing opinion this is not contradicted by the anatomical evidence (Groves & Paterson, 1991). This implies that the African hominoids first split into Pan-Homo (smaller, relatively gracile) and Gorilla (larger, super-robust), and that many of the traits that common chimps share with gorillas but not with bonobos or humans could have developed in parallel with gorillas (e.g. very long and sexually dimorphic canines, “very” dorsal foramen magnum, ectocranial crests, arms considerably longer than legs). Convergent and parallel, even reverse or fluctuating evolution of anatomical traits are among the commonest features of biological evolution (e.g. Trinkaus, 1990; Hartman, 1989; Sheldon, 1988; Seger, 1987; Gibbs & Grant, 1987; Cartmill, 1982; White & Harris, 1977; Darwin, 1903, p.171), and the final proof that Darwinism is not a tautology. “Parallel evolution occurs when two species adopt a lifestyle that is more or less similar. If the lifestyle is essentially identical, and the species from a similar genetic background, the end result may be almost indistinguishable to other than detailed examination” (R. G. Silson, pers. comm.). The very long canines and very dorsal foramen magnum of adult gorilla and common chimp males (but not of subadult African apes nor of adult bonobos) could well be derived and rather recent adaptations to the same environmental (e.g. in response to climatic) changes and cannot be explained by mere allometry. Even knuckle-walking of chimps and gorillas has been argued to have arisen independently (Begun, 1992), possibly in more bipedal ancestors (Kleindienst, 1975; Hasegawa et al., 1985; Edelstein, 1987). Indeed, Gorilla knuckle-walking anatomy and ontogeny are much better developed than in Pan, and are different from Pan (Inouye, 1992). And the LCA (the last common ancestor of Homo and Pan) had not yet acquired knuckle-walking since humans do not at any age show the slightest trace of knucklewalking behaviour: (1) we lean (e.g. on a table) far more comfortably on our proximal than on our middle hand phalanges; (2) whereas in knuckle-walking apes the middle hand phalanges are naked, in many men they are dorsally haired, and fingers III and IV (that bear most weight in knuckle-walkers) even more frequently than V and II (Harrison, 1958; Singh, 1982; Ikoma, 1986); (3) “human infants walk or run spontaneously on all fours and this invariably with the palms flat on the ground and the fingers completely extended” (Schultz, 1936, p. 264). Lucy’s arms were much shorter than a bonobo's (humerus 24 cm vs 29 cm; cf. 26 cm in human pygmies) and lacked knuckle-walking adaptations (Jungers, 1982; Stern & Susman, 1982), but later the small hominid O.H.62 had more chimp- and bonobo-like proportions (Korey, 1990; Aiello & Dean, 1990, p. 258; Wood, 1992, box 2), and the larger KNM-ER 1500 (probably a boisei female) showed some gorilla-like proportions, e.g. relatively large forelimbs (McHenry, 1978, 1992). While the early KNM-KP 271 distal humerus was “similar to that of modern man” (Senut, 1980; cf. Oxnard, 1984, fig.10.12; and Aiello & Dean, 1990, p. 365 and p. 368), A. robustus TM 1517 was more chimp-like, and A. boisei KNM-ER 739 more gorilla-like (Senut, 1980; Aiello & Dean, 1990, pp. 365-368). Body weight estimations for robustus and boisei based on formulae for ape postcrania fit much better with the massive jaws than estimations based on human formulae (see McHenry, 1991). The boisei ulnae O.H.36 and L.40-19 and humerus KNM-ER 739 were of gorilla robusticity and length (McHenry, 1991, 1992; Howell & Wood, 1974; Senut, 1980; Leakey, 1971; Aiello & Dean, 1990, p. 367-369), and the curvature and the cross-section of L.40-19 are reminiscent of knuckle-walkers (Howell & Wood, 1974); “the Rudolf australopithecines, in fact, may have been close to the ‘knuckle-walker’ condition, not unlike the extant African apes” (Leakey, 1971). Their arm lengthening and strengthening is paralleled ontogenetically in the African apes; Rensch (1972, p. 45) even states that “it is only after birth that an ape’s arms become disproportionally long”, but this can only be true when arm growth relative to the height in African apes is compared with monkeys (Schultz, 1936, fig. 15). The possibility should be considered that robustus and boisei did not belong to the same (robust) branch, but that their robust traits represented parallel adaptations (cf. Delson, 1987; Grine, 1987; Trinkaus, 1990; Conroy, 1990, fig. 6.40.d). Indeed, super-robust specimens from East Africa (KNM-WT 17000) appeared in the fossil record before the less robust A. robustus from South Africa, and the morphological differences between africanus and robustus are less than those between robustus and boisei (e.g. Leakey, 1959, 1960; Wood, 1978; Wood & Chamberlain, 1987). This is particularly clear in dental morphology (Hunt & Vitzthum, 1986; Wood & Uytterschaut, 1987; Wood & Engleman, 1988; Wood et al., 1988). An analysis of root morphology in mandibular premolars, for instance, revealed moderate root reduction in A. africanus, A. robustus and P. troglodytes, pronounced reduction in Homo but root molarization in A. boisei compared with A. afarensis, G. gorilla and most higher primates (Wood et al., 1988). Possible evolutionary trees of the australopithecines are obscured by the incompleteness of the fossil material, by parallel (e.g. boisei/robustus) or even reverse evolution of some anatomical characters, by mosaic evolution and retention of ancestral characters in some branches (stagnations, and “sudden” accelerations of certain features). Nevertheless, some relationships seem to emerge (Figure 1): (1) In East Africa, A. boisei – and perhaps some larger afarensis from A.L.333 or Laetoli as well – is morphologically (Tables 2 and 4), and therefore probably cladistically closer to Gorilla than to Pan or Homo. (This does not imply that some of their anatomical features cannot be closer to humans or to chimpanzees than to gorillas. Nor that (all) gorillas must descend from A. boisei. Biomolecular data suggest that the difference between highland and lowland gorilla - like that between common chimp and bonobo - is less than half that between man and chimp (Gribbin & Cherfas, 1983, p. 137), i.e. highland and lowland gorillas possibly diverged 3-2 Myr BP. In view of the small anterior dentition of A. boisei, the possibilities should be considered that some or all gorillas descend from a form nearer to KNM-WT 17000 than to A. boisei, or - the prevailing opinion - that fossil ancestors of gorillas have not been discovered yet.) Since Homo and Pan diverged probably one or two million years later than Pan-Homo and Gorilla (e.g. Ruvolo et al., 1991), it is not surprising that Wood (1978), in a classification of East African fossil hominids, states that “by relying solely on morphology, the taxa presented are most obviously subdivided into the ‘robust’ australopithecine taxon Australopithecus boisei, and another group consisting of all the remaining taxa... In contrast to the conformity within the ‘robust’ lineage the ‘non-robust’ hominids display a wide range of variation”. (2) South African australopiths (Tables 3 and 4) - and probably some very small specimens from East Africa such as Lucy or “H. habilis” as well (cf. Zihlman, 1985; Ferguson, 1987a,b, 1992; Wood, 1978, 1992a,b) - show more affinities with Pan-Homo than with Gorilla. A striking example is the incus bone SK 848, which is clearly more like Homo or Pan than like Gorilla (Rak & Clark, 1979, fig. 1). Because A. robustus lived at the time of KNM-ER 1470 (probably an early Homo), and Taung lived even later (Partridge, 1973, 1985), they could have belonged to the Pan clade but not to the Homo clade. Taung’s endocast, dentition, facial growth and possibly foramen magnum position strikingly resemble those of apes and chimpanzees (Falk et al., 1989). Simons (1989): “Dart’s enthusiasm for A. africanus as a human ancestor was occasioned by his misidentification of the lamboid structure as the lunate sulcus and thus reading a human-like sulcal pattern in the natural endocast of the brain of the Taung child”. Discussion Why are many paleoanthropologists so reluctant to consider just the possibility that some or all of the australopithecines could have been evolutionarily nearer to one of the African apes than to humans? (1) When paleoanthropologists discover fossil remains, they often - understandably tend to stress the human-like features of their finds. Subsequent researchers, however, frequently obtain more detached views. (2) Man is often considered to possess a great number of features that are uniquely derived from the supposed “primitive hominoid condition”: thick enamel, short canines, forward position of the foramen magnum, short ilia, non-grasping feet, low intermembral index, etc. But the primitive hominoid condition is largely hypothetical: as discussed above, man seems to be more primitive in some of these features than the apes (e.g. thick enamel, low pelvis); and in many features the differences between the ape species (e.g. between Pongo and Gorilla) are larger than those between humans and some of the apes (e.g. relative arm length, foot shape). Most probably the ancestral hominoids were neither like humans nor like any of the extant ape species. (3) In the same way, it is often uncritically accepted that the LCA was much more chimp- than human-like. As Hasegawa et al. (1985) say: “It is unknown whether the last common ancestor of human and chimpanzee was like the living chimpanzee or like the living human. However it seems to have been widely assumed implicitly that the common ancestor of the two species was more like the chimpanzee than the human. There has been a tendency to view hominid features as specialized and those of apes as unspecialized. Any fossil hominoids that bear some resemblance to humans have been readily considered to be human ancestors”. Such assumptions are reinforced by using terms like “primitive”, “plesiomorphic” or “less advanced” (which imply that the ancestral character is known), where the more neutral “apelike” or (if possible, and more precisely) “chimp-like”, “bonobo-like” or “gorilla-like” would be preferable. The anthropocentric fallacy enshrined in the usage of “primitive” has more than once been challenged (Gribbin & Cherfas, 1993; Edelstein, 1987; Verhaegen, 1990). Since homoplasy, convergence, and reverse (and even fluctuating) evolution are so common, ontogeny may provide more reliable criteria to decide what is primitive (Trinkaus, 1990; cf. Northcutt, 1990). “Morphological characters can be subjected to parallel or convergent evolution, and cannot be used with confidence for phylogenetic reconstructions unless the probability of parallel evolution is evaluated or rejected in a proper way” (Hasegawa et al., 1987). As we go further back in time, we may expect that human ancestors become more chimpanzee-like, but also that the chimpanzees’ ancestors become more human-like, i.e. display a few human-like features. Assuming that the LCA looked much more like a chimpanzee than a human and that subsequently humans have evolved much more than common chimps is statistically less likely than assuming that the LCA already possessed a few mosaic human-like features (e.g. facultative bipedalism, orthognathism, thicker enamel) and that both branches (Homo and Pan) underwent evolutionary changes towards their present-day representants (e.g. much longer legs in humans, longer arms in chimps). In fact, it seems most economical to assume that the LCA 8-4 Myr BP looked somewhat like bonobos (or like subadult chimpanzees), which are in several instances - but not, for instance, in body weight - intermediate between humans and common chimps, e.g. in relative canine size, canine dimorphism, orthognathisrn, foramen magnum indices, relative arm and leg lengths, bipedalism and knuckle-walking. Although the LCA lived earlier, the gracile australopiths of 3-2.5 Myr BP (Lucy, Sterkfontein) are the best approximation we presently have: see Aiello & Dean (1990, p. 254), or in Table 3 the quotation of Zihlman et al. (1978). It seems that, while our ancestors were becoming more and more human-like, the African apes - at first the ancestor of the gorillas and shortly thereafter that of both chimpanzees - for unknown reasons (climatic and habitat changes?) - broke away from our evolutionary direction, partially reversed their evolution, and became again - the three species to different degrees - more like monkeys in thinner enamel, larger front teeth, prognathism, ectocranial crests, relatively smaller endocast, more dorsal foramen magnum, elongated iliac blades, short femoral necks, less valgus knees, more grasping feet, quadrupedalism, etc. (but not, for instance, in body size, relative arm length, knuckle-walking, pelvic height, number of lumbar, sacral and coccygal vertebrae). There are admittedly several weak spots in this scenario: the many reversals (notably in the lower limb anatomy) and parallelisms (e.g. anterior dentition, iliac anatomy, knucklewalking adaptations) in the evolution of Gorilla and Pan. (If Pongo is included in the comparison, even more - apparently improbable - parallelisms are needed, although, as discussed above, for most of such features (e.g. sexual dimorphism, foramen magnum position, relative arm length, foot shape), at least one African ape species can be found to be more different from orangs than from humans, and Andrews (1992), in a review of Miocene hominoids, even asserts that “if Sivapithecus belongs in the orangutan clade, as I have argued, the shared [postcranial] morphology of the orang-utan and the African apes must have arisen independently”). However, if these reversals and parallelisms are correlated (re-adaptations to, for instance, an older, less “innovating” or less human-like lifestyle or environment), the counterargument to my scenario fails. Moreover, the traditional hypothesis - that all australopithecines are more closely related to humans than to African apes - seems to have more serious difficulties, since it does not explain: (1) the apparent complete absence of fossil ancestors or relatives of any African ape; (2) the various australopith-like features that are present in premature though not in adult African apes (e.g. orthognathy, less dorsal foramen magnum, more humanlike feet); (3) the fact that all australopiths lack the uniquely derived bony features which set man (at least since H. erectus) clearly apart from the other catarrhines (e.g. external nose, very large brain, very long legs), and that they resemble the apes in these respects; (4) that every one of the australopith species has more features in common with either gorillas or chimpanzees than with humans (e.g. Tables 2, 3 and 4); (5) and that at the time of the robust australopiths there already lived more humanlike creatures (KNM-ER 1470). (Oxnard’s (1984, p. 307-332) proposition - that the australopiths were evolutionarily nearly equidistant from African apes and humans and left no descendants today - does not have the fifth difficulty. Also, in my opinion, Oxnard correctly states that many australopith postcrania were biomechanically unique, and could have represented adaptations to a welldefined lifestyle (e.g. Verhaegen, 1992).) Conclusion A review of the paleo-anthropological literature reveals no data that exclude the possibility that both gorillas and chimpanzees could have had australopith ancestors. Bipedalism is generally considered to be the shared feature that links australopithecines with humans, and there is no doubt that at least some of the australopith species were partial bipeds. But it has never been proven that the African apes’ unique locomotion (plantigrade knuckle-walking) could not have evolved from some kind of (“short”-legged) bipedalism. In fact, insofar as the fragmentary fossil material and the incomplete comparisons with extant apes allow, ontogenetic and morphological evidence tends to favour the hypothesis that the last common ancestor of Homo and Pan 8-4 Myr BP was a partially bipedal, gracile australopith with chiefly a mosaic of human and chimpanzee (esp. bonobo) features: low sexual dimorphism, minimal prognathism, slightly enlarged canines, non-protruding nasal skeleton, smooth ectocranium without crests, “small” brain with ape-like sulcal pattern, relatively non-flexed basicranium, intermediate position of foramen magnum, “short” forelimbs without knucklewalking features, low ilia, (very) long femoral necks, “short” legs, (very) valgus knees, full plantigrady, longer and not very abductable halluces. I expect that when australopith fossil material is re-examined and compared in detail with every one of the large hominoids, in most cases it will resemble either Pan or Gorilla more closely than it resembles Homo and certainly Pongo. ACKNOWLEDGMENTS – I wish to thank A. S. Ryan, M. Goodman, M. Hasegawa, R. G. Silson, M. R. Kleindienst, E. Morgan and especially J. Verhulst for their corrections and comments on various versions of this manuscript. References Aiello L. & Dean C., 1990. An Introduction to Human Evolutionary Anatomy. Academic Press, London. Andrews P., 1992. Evolution and environment in the Hominoidea. Nature, 360: 641-646. Andrews P. & Cronin J.E., 1982. The relationships of Sivapithecus and Ramapithecus and the evolution orang-utan. Nature, 297: 541-546. Ashton E.H. & Zuckerman S., 1952. Age changes in the position of the occipital condyles in the gorilla. American Journal of Physical Anthropology, 10: 277-288. Begun D. R., 1992. Miocene fossil hominids and the chimp-human clade. Science, 257: 19291933. Beynon A. D. & Wood B. A., 1986. Variations in enamel thickness and structure in East African hominids. American Journal of Physical Anthropology, 70: 177-193. Beynon A. D., Dean M.C. & Reid D. J., 1991. On thick and thin enamel in hominoids. American Physical Anthropology, 86: 295-309. Bromage T. G., 1985. Taung facial remodelling: a growth and development study. In (P. V. Tobias, Evolution, pp. 239-245, Alan R. Liss, New York. Bromage T. G. & Dean M. C., 1985. Re-evaluation of age at death of immature fossil hominids. Nature, 317: 527. Broom R. & Robinson J. T., 1950. Notes on the pelvis of the fossil ape-men. American Journal of Physical Anthropology, 8:489-494. Caccone A. & Powell J. R., 1989. DNA divergence among hominoids. Evolution, 43: 25-942. Calcagno J.M. & Gibson K. R., 1988. Human dental reduction: natural selection or the Probable Mutation Effect. American Journal of Physical Anthropology, 77: 505-517. Cartmill M., 1982. Basic primatology and prosimian evolution. In (F. Spencer, ed. A History of Physical Anthropology 1930-1980, pp. 14-186, Academic, New York. Cave A.J.E. & Wheeler Haines R., 1940. The paranasal sinuses of the anthropoid apes. Journal of Anatomy, 43-523. Conroy G. C., 1990. Primate Evolution. W.W. Norton, New York. Coon S.C., 1954. The Story of Man. Knopff, New York. Dart R. A., 1925. Australopithecus africanus: the man-ape of South America. Nature, 115, 195- 199. Darwin C., 1903. On the Origin of Species by Means of Natural Selection. Watts and Co, London. Delson E., 1987. Evolution and palaeobiology of robust Australopithecus. Nature, 327: 654655. Demes B. & Creel N., 1988. Bite force, diet and cranial morphology of fossil hominids. Journal of Human Evolution, 17: 657-670. De Waal F., 1988. Peacemaking among Primates. Harvard University Press, London. Duckworth W.L.H., 1925. The fossil ape from Taungs. Nature, 115: 236-237. Eckhardt R.B., 1987. Hominoid nasal region polymorphism and its phylogenetic significance. Nature, 328:333-335. Edelstein S. J., 1987. An alternative paradigm for hominoid evolution. Human Evolution, 2: 169-174. Falk D., 1985. Hadar AL 162-28 endocast as evidence that brain enlargement preceded cortical reorganization in hominid evolution. Nature, 313: 45-47. Falk D., 1987. Hominid paleoneurology. Annual Review of Anthropology, 16: 13-30. Falk D., Hildeholt C. & Vannier M. W., 1989. Reassessment of the Taung early hominid from a perspective. Journal of Human Evolution, 18: 485-492. Ferguson W.W., 1987a. Revision of the subspecies of Australopithecus africanus (Primates, Hominidae), including a new subspecies from the late Pliocene of Ethiopia. Primates, 28: 258-265. Ferguson W.W., 1987b. Reconstruction and re-evaluation of the skull of Homo antiquus (Hominoidea, Homininae) from Hadar. Primates, 28: 377-391. Ferguson W.W., 1989a. Reappraisal of the taxonomic status o the cranium Stw 53 of the PlioPleistocene from Sterkfontein, in South Africa. Primates, 30: 103-109. Ferguson W.W., 1989b. A new species of the genus Australopithecus (Primates – Hominidae) from the Plio-Pleistocene deposits West of Lake Turkana in Kenya. Primates, 30: 223232. Ferguson W. W., 1992. “Australopithecus afarensis”: a composite species. Primates, 33: 273-279. Franciscus R. G. & Trinkaus E., 1988. Nasal morphology and the emergence of Homo erectus. American Journal of Physical Anthropology, 75: 517-527. Gebo D. L., 1992. Plantigrady and foot adaptation in African apes: implications for hominid origins. American Journal of Physical Anthropology, 89: 29-58. Gibbs H. L. & Grant P. R., 1987. Oscillating selection on Darwin’s finches. Nature, 327: 511513. Gonzalez I. L., Sylvester J. E., Smith T. F., Stamholian D. & Schmickel R. D., 1990. Ribosomal RNA gene sequences and hominoid phylogeny. Molecular Biological Evolution, 7: 203-219. Goodman M., 1982. Biomolecular evidence on human origins from the standpoint of Darwinian theory. Human Biology, 54: 247-64. Gribbin J. & Cherfas J., 1983. The Monkey Puzzle. Triad, London. Grine F. E., 1987. The evolutionary history of the “robust” australopithecines. AnthroQuest, 38: 7-9. Groves C. P. & Paterson J. D., 1989. Testing hominoid phylogeny with the PHYLIP programs. Journal of Human Evolution, 20: 167-183. Harrison R. J., 1958. Man the Peculiar Animal. Penguin, Harmondsworth. Hartman S. E., 1989. Stereophotogrammatic analysis of occlusal morphology of extant hominoid molars: phenetics and function. American Journal of Physical Anthropology, 80:145-166. Hasegawa M., Kishino H. & Yano T., 1985. Dating of the human-ape splitting by a molecular clock mitochondrial DNA. Journal of Molecular Evolution, 2:160-174. Hasegawa M., Kishino H. & Yano T., 1987. Man’s Place in Hominoidea as inferred from molecular clocks. Journal of Molecular Evolution, 26: 13-147. Hasegawa M., Kishino H. & Yano T., 1988. Phylogenetic inference from DNA sequence data. In (K. Matsusita, ed.) Statistical Theory and Data Analysis II, pp. 1-13, NorthHolland, Amsterdam. Hasegawa M., Kishino H. & Yano T., 1989. Estimation of branching dates among primates by molecular clocks of nuclear DNA which slowed down in Hominoidea. Journal of Human Evolution, 18: 461-476. Howell F. C. & Wood B. A., 1974. Early hominid ulna from the Omo basin, Ethiopia. Nature, 249: 174-6. Hunt K. & Vitzthum V. J., 1986. Dental metric assessment of the Omo fossils: implications for the phylogenetic position of Australopithecus africanus. American Journal of Physical Anthropology,71:141-155. Ikoma E., 1986. Anthropological study of digital and parietal hair of Canadians. American Journal of Physical Anthropology, 69: 483-487. Inouye S. E., 1992. Ontogeny of knuckle-walking hand postures in African apes. American Journal of Physical Anthropology, Supplement 14: 55. Jenkins L., 1991. Synapomorphic features in Gorilla gorilla, Pan troglodytes and Pliopleistocene hominids. American Journal of Physical Anthropology, Abstracts 60th annual meeting, p. 100. Johanson D. C. & Edey M. A., 1981. Lucy, the Beginnings of Mankind. Granada, London. Jungers W. L., 1982. Lucy’s limbs: skeletal allometry and locomotion in Australopithecus afarensis. Nature, 297: 676-678. Keith A., 1925a. The fossil anthropoid ape from Taungs. Nature, 115: 234-235. Keith A., 1925b. The Taungs skull. Nature, 116: 462-463. Keith A., 1931. New Discoveries Relating to the Antiquity of Man. Williams and Norate, London. Kennedy G. E., 1991. On the autapomorphic traits of Homo erectus. Journal of Human Evolution, 20: 375-412. Kimbel W. H., White T. D. & Johanson D. C., 1984. Cranial morphology of Australopithecus afarensis: a comparative study based on a composite reconstruction of the adult skull. American Journal of Physical Anthropology, 64: 337-388. Kinzey W. G., 1970. Basic rectangle of the mandible. Nature, 228: 289-290. Kleindienst M. R., 1975. On new perspectives on ape and human evolution. Current Anthropology. 16: 644-646. Korey K. A., 1990. Deconstructing reconstruction: the OH 62 humerofemoral index. American Journal of Physical Anthropology, 83:25-33. Kortlandt A., 1975. On new perspectives on ape and human evolution: reply. Current Anthropology, 16: 647- 651. Laitman J. T. & Heimbuch R. C., 1982. The basicranium of Plio-Pleistocene hominid as an indicator of their upper respiratory systems. American Journal of Physical Anthropology, 59: 323-343. Latimer B., Ohman J.C. & Lovejoy C. O., 1987. Talocrural joint in African hominoids: implications for Australopithecus afarensis. American Journal of Physical Anthropology, 74: 155-175. Leakey L. S. B., 1959. A new fossil skull from Olduvai. Nature, 184: 491-493. Leakey L. S. B., 1960. The affinities of the new Olduvai australopithecine. Nature, 186: 456458. Leakey R. E., 1971. Further evidence of Lower Pleistocene hominids from East Rudolf, North Kenya. Nature, 231: 242-245. Leakey R. E., 1981. The Making of Mankind. Michael Joseph, London. Leakey R. E. F. & Walker A., 1988. New Australopithecus boisei specimens from East and West Lake Turkana, Kenya. American Journal of Physical Anthropology, 76: 1-24. Le Gros Clark W. E., 1978. The Fossil Evidence for Human Evolution. University of Chicago Press, Chicago. Lewin R., 1987. Bones of Contention. Simon & Schuster, New York. Martin L., 1985. Significance of enamel thickness in hominoid evolution. Nature, 314: 260263. Masters A. V., Falk D. & Gage T. B., 1991. Effects of age and gender on the location and orientation of the foramen magnum in rhesus macaques (Macaca mulata). American Journal of Physical Anthropology, 86: 75-80. McHenry H. M., 1978. Fore- and hindlimb proportions in Plio-Pleistocene hominids. American Journal of Physical Anthropology, 49: 15-22. McHenry H. M., 1983. The capitate of Australopithecus afarensis and A. africanus. American Journal of Physical Anthropology, 62: 187-198. McHenry H. M., 1991. Petite bodies of the “robust” australopithecines. American Journal of Physical Anthropology, 86: 445-454. McHenry H. M., 1992. Body size and proportions in early hominids. American Journal of Physical Anthropology, 87: 407-431. Nishida T., 1980. Local differences to water among wild chimpanzees. Folia Primatologica, 33: 189-209. Northcutt R.G., 1990. Ontogeny and phylogeny: a re-evaluation of conceptual relationships and some applications. Brain, Behavior and Evolution, 36: 116-140. Oxnard C. E., 1984. The Order of Man. Yale University Press, New Haven. Partridge T. C., 1973. Geomorphological dating of openings at Makapansgat, Sterkfontein, Swartkrans and Taung. Nature, 246: 75-79. Partridge T.C., 1985. Spring flow and tufa accretion at Taung. In (P.J. Tobias, ed.) Hominid Evolution, pp. 171- 187. Alan R. Liss, New York. Pilbeam D., 1982. New hominoid skull material from the Miocene of Pakistan. Nature, 295: 232-234. Rak Y. & Clarke R. J., 1979. Ear ossicle of Australopithecus robustus. Nature, 279: 62-63. Rak Y. & Howell F.C., 1978. Cranium of a juvenile Australopithecus boisei from the Lower Omo Basin, Ethiopia. American Journal of Physical Anthropology, 48: 345-366. Reader J., 1988. Missing Links. Penguin, London. Rensch B., 1972. Homo sapiens, from Man to Semigod. Columbia University Press, New York. Robinson J. T., 1960. The affinities of the new Olduvai specimen. Nature, 186: 456-458. Robinson J. T., 1972. The bearing of East Rudolf fossils on early hominid systematics. Nature, 240: 239-240. Ruvolo M., Disotell T. R., Allard M. W., Brown W. M. & Honeycutt R. L., 1991. Resolution of the African hominoid trichotomy by use of a mitochondrial gene sequence. Proceedings of the National Academy of Sciences, U.S.A., 88: 1570-1574. Ryan A. S. & Johanson D. C., 1989. Anterior dental microwear in Australopithecus afarensis: comparisons with human and nonhuman primates. Journal of Human Evolution, 18: 235-268. Sarich V. M., 1977. Rates, sample sizes, and the neutrality hypothesis for electrophoresis in evolutionary studies. Nature, 265: 24-28. Sarich V.M. & Wilson A. C., 1967. Immunological time scale for hominid evolution. Science, 158: 1200-1203. Schmid P. & Stratil Z., 1986. Growth changes, variations and sexual dimorphism of the gorilla skull. In (J.B. Else & P.C. Lee, eds.) Primate Evolution, pp. 239-247, Cambridge University Press, Cambridge. Schoenemann P. T., 1989. Comparison of intraspecific craniometric variability in Homo, Pan and Gorilla. American Journal of Physical Anthropology, 78: 298. Schultz A. H., 1936. Characters common to higher primates and characters specific for man. Quarternary Review of Biology, 11: 259-283 and 425-455. Schultz A. H., 1941. Ontogenetic specializations of man. Archiv Julius Klaus-Stiftung, XXIV: 197-216. Schultz A. H., 1941. Growth and development of the orang-utan. Contributions to Embryology, XXIX: 57-1 10. Schultz A. H., 1950. The physical distinctions of man. Proceedings of the American Philosophical Society, 94: 428-449. Schultz A. H., 1955. The position of the occipital condyles and of the face relative to the skull base in primates. American Journal of Physical Anthropology, 13: 97-120. Seger J., 1987. El Nino and Darwin’s finches. Nature, 327: 461. Senut B., 1980. New data on the humerus and its joints in Plio-Pleistocene hominids. Collegium Anthropologicum, 4: 87-93. Sheldon P., 1988. Making most of the evolution diary. New Scientist, 1596: 52-54. Silson R. G., 1988. Additive Gene Systems. Greenfield Publications, Tring. Sibley E. G., Comstock J.A. & Ahlquist E. G., 1990. DNA hybridization evidence of hominoid phylogeny - a reanalysis of data. Journal of Molecular Evolution, 30: 202-236. Simons E. L., 1989. Human origins. Science, 245, 1343-1350. Singh J.D., 1982. Distribution of hair on phalanges of the hand in Nigerians. Acta Anatomica, 112: 31-35. Smith G.E., 1925. The fossil anthropoid ape from Taungs. Nature, 1 15: 235. Stem J. T. & Susman R.L., 1983. The locomotor anatomy of Australopithecus afarensis. American Journal of Physical Anthropology, 60: 279-317. Tobias P. V., 1968. Pleistocene deposits and new fossil localities in Kenya. Nature, 217: 479480. Trevino S., 1991. Grain-collection, Humans' Natural Ecological Niche. Vantage Press, New York. Trinkaus E., 1990. Cladistics and the hominid fossil record. American Journal of Physical Anthropology, 83: 1-11. Verhaegen M. J. B., 1990. African ape ancestry. Human Evolution, 5: 295-297. Verhaegen M. J. B., 1992. Did robust australopithecines partly feed on hard parts of Gramineae? Human Evolution, 7: 63-64. Vigilant L., Stoneking M., Harpending H., Hawkes K. & Wilson A. C., 1991. African populations and the evolution of human mitochondrial DNA. Science, 53: 1503-7. Walker A., Leakey R. E., Harris J. M. & Brown F. H., 1986. 2.5-Myr Australopithecus boisei from West of Lake Turkana, Kenya. Nature, 322: 517-552. White T. D. & Harris J. M., 1977. Suid evolution and correlation of African hominid localities. Science, 198: 13-21. Wood B. A., 1978. Classification and phylogeny of East African hominids. In (P. V. Tobias, ed.) Hominid Evolution, pp. 351-372, Alan R. Liss, New York. Wood B. A., 1992a. Old bones match old stones. Nature, 355: 678-679. Wood B. A., 1992b. Origin and evolution of the genus Homo. Nature, 355: 783-790. Wood B. A., Abbott S. A. & Uytterschaut H., 1988. Analysis of the dental morphology of Plio-Pleistocene hominids. IV. Mandibular postcanine root morphology. Journal of Anatomy, 156: 107-139. Wood B. A. & Chamberlain A. T., 1987. The nature, and affinities of the "robust" australopithecines: a review. Journal of Human Evolution, 16: 625-641. Wood B. A. & Engleman C.A., 1988. Analysis of the dental morphology of Plio-Pleistocene hominids: V. Maxillary postcanine tooth morphology. Journal of Anatomy, 161: 1-35. Wood, B. A. & Uytterschaut H., 1987. Analysis of the dental morphology of Plio-Pleistocene hominids: III. Mandibular premolar crowns. Journal of Anatomy, 154: 121-156. Woodward A. S., 1925. The fossil anthropoid ape from Taungs. Nature, 115: 235-236. Zihlman A. L, Cronin J.E, Cramer D. L. & Sarich V.M., 1978. Pygmy chimpanzee as a possible prototype for the common ancestor of humans, chimpanzees and gorillas. Nature, 275: 744-746. Zihlman A. L., 1985. Australopithecus afarensis: two sexes or two species? In (P. V. Tobias, ed.) Hominid Evolution, pp. 213-220, Alan R. Liss, New York. Table 4A - Measurements of australopithecine, African ape and human skulls foramen magnum indicesa - basion I - basion II - opisthion I - opistbion II 333-45 (51.4) (77.2) (15.9) (21.3) Sts.5 43.7 66.2 (14.2) (19.4) frontal bene indicesb - B at post-orb.constrict.(mm) - minimum frontal B (mm) - fronto-temporal B index - superior facial B (mm) - inner biorbital B (mm) - fronto-facial B index - fronto-biorbital B index recon. 66.0 107.0 94.0 62.0 78.6 Sts.5 64.0 48.0 75.0 93.5 84.2 68.4 76.0 rel.H ant.masseter originc - zygomax.-alveolar margin - orbitoalveolar H - zm-alv/lorb-alv.index recon. 24.0 (47.5) 50. 5 mandibulad - ramus H (mm) - ramus B (mm) - H/B % recon. 55.0 55.0 100.0 mandibular fossa e - L (mm) - B (mm) - D (mm) - L/B % - D/L % - D/B % bite forcef - infratemporal fossa (cm²) - molar crown are (cm²) - bite force equivalent M² - bite force equivalent at I recon. (16.0) 5.38 13.9 9.2 incisal microwearg - wear striae (/mm²) - pits (/mm²) - pit diameter (mm) - wear striation orientation A.afar. 4.40 2.17 .07 61° O.H.5 54.9 69.3 27.6 33.3 ER-406 40.5 (57.1) 15.6 (20.6) SK-48 (67.0) (27.0) 40.3 (100.0) (93.0) 65.7 72.0 O.H.5 69.4 (25.0) 36.0 115.5 97.0 60.3 71.5 ER-406 62.0 31.0 50.0 114.0 100.0 54.4 62.0 Gorilla 69.0 44.2 64.1 125.3 105.1 55.5 66.0 Pt 70.5 54.7 77.4 106.2 90.0 66.6 78.4 Peking 96.0 86.0 89.6 121.0 111.0 79.3 86.5 Sts.5 32.0 51.0 62.7 SK-48 38.3 61.0 62.8 O.H.5 36.0 75.1 47.9 ER-406 40.0 62.0 64.5 Gorilla 36.4 71.3 51.2 Pt 24.6 51.2 48.0 Homo 18.1 41.3 43.8 Sts.7 61.0 46.8 130.0 SK 60.7 54.5 111.4 O.H.5 65.0 - Natron 47.0 52.8 89.0 Gorilla 67.0 62.5 108.0 Pt 43.1 46.4 92.9 Homo 35.9 37.4 94.7 MLD 22.7 30.4 7.8 74.6 34.2 25.5 A.rob. 26.7 31.9 9.5 83.5 35.8 29.8 O.H.5 27.8 34.4 8.7 81.1 31.2 25.2 G male 27 46 10 58.7 37.1 22.1 Pt male 25 29 7 86.3 27.9 24.1 Homo 25.0 23.8 14.5 99.0 61.4 60.8 Sts.5 9.7 5.87 7.8 5.3 SK-48 (11.0) 5.73 12.2 9.O Gorilla 17.5 6.42 15.6 9.4 Pt 12.7 3.53 10.9 6.5 Gorilla 3.02 1.87 .06 60° Pt 5.27 3.87 .06 37° ER-406 18.3 8.92 18.0 12.0 Pt 33.3 52.0 11.2 16.2 Pp 41.6 61.3 16.3 22.1 Pp 7.5 2.45 6.6 3.9 Peking 81.1 87.2 31.8 33.5 Homo 7.2 2.86 6.7 4.3 Eskimo 6.85 2.17 .18 17° Table 4A-4B - Legend H human(like); Pt common chimp(like); Pp pygmy chimp(like); G gorilla(like); G>P, P>G apelike; Pp? like Pt, but possibly even more like Pp (no figures available for Pp); + very much like ... ; - well outside African hominoid range; B breadth; D depth; H height; L length; recon. reconstruction of large A.afarensis; 333-45 large A.afarensis from A.L.333-45; Sts.5, Sts.7 A.africanus from Sterkfontein; MLD A.africanus MLD-37138 from Makapansgat; SK mean of A.robustus SK-12, SK-23 and SK-34 from Swartkrans; O.H.5, ER-406 A.boisoi from Olduvai and Turkana; Natron from Peninj River; Peking H.erectus; Eskimo H.sapiens. In extant hominoids, measurements are means of males and females, unless mentioned otherwise. a e b f Kimbel et al., 1984, table 9 ibid., table 6 c ibid., table 5 d ibid., table 2 Tobias, 1968, table 1 Demes & Creel, 1988, table 1 and 2 g Ryan et al., 1989, table 21 Table 4B - Australopiths compared with African hominoids foramen magnum indicesa - basion I - basion II - opisthion I - opistbion II 333-45 Sts.5 Pp Pp+ He Pp Pp+ Pp Pp+ Pp>Pt frontal bene indicesb - B at post-orb.constriction - minimum frontal B (mm) - fronto-temporal B index - superior facial B (mm) - inner biorbital B (mm) - fronto-facial B index - fronto-biorbital B index recon. Sts.5 SK-48 O.H.5 ER-406 G>P G>P G>P+ G>P+ G>P G>P GGGP+ GGGP+ Pp? P H>PG H>PG Pp? Pp? P P>G G>P P+ P+ G>P G>P G+ (Pp??) rel.H ant.masseter originc - zygomax.-alveolar margin - orbitoalveolar H - zm-alv/lorb-alv.index recon. P+ P G+ (Pp?) mandibulad - ramus H (mm) - ramus B (mm) - H/B % recon. Sts.7 G>P G G>P P+ H>PG G- mandibular fossa e - L (mm) - B (mm) - D (mm) - L/B % - D/L % - D/B % Sts.5 G>P P+ G>P- O.H.5 Pp Pp He He+ SK-48 G+ P>G G>P- 0.H.5 G+ G P+ ER-406 Pp+ Pp>Pt Pp+ Pp ER-406 GG>P G>P- SK 0.H.5 Natron G G+ P G>P P>G G+ P>H MLD A.rob. O.H.5 H=P G G P+ P+ P P G+ G>P P P+ P G>P+ G+ P P>G+ P>G+ P>G+ bite forcef - infratemporal fossa (cm²) - molar crown area (cm²) - bite force equivalent M² - bite force equivalent at I recon. G G G G+ incisal microwearg - wear striae (/mm²) - pits (/mm²) - pit diameter (mm) - wear striation orientation A.afar. P>G H+ G>P+ G+ Sts.5 Pp>t G HP H>P SK-48 Pt G Pt>G G+ (Gorilla??) (Pp??) (Pp??) ER-406 G+ GG G- (all) (Pp??) Figure 1 - An example of a possible evolutionary tree of fossil hominids 0 Myr BP Gg : : : ? 1 2 3 4 Ab Ab Ab Ab Pt : : : : ? Ar Ar Pp : : : : : ? O.H.62 . . WT-17000 : : ?A.L.333 : : ?Laetoli Hs : Hn He He He He He . . ?ER-1470 . . . At ? At ? ?Lucy . . ?BC-1 . . Gorilla gorilla Pt Pan troglodytes Pp Pan paniscus Ab Australopithecus boisei Ar A. robustus At A. africanus transvaalensis He Homo erectus Hn Homo neanderthalensis Hs Homo sapiens sapiens DID ROBUST AUSTRALOPITHECINES PARTLY FEED ON HARD PARTS OF GRAMINEAE? Human Evolution 7: 63-64, 1992 Estimates of bite force suggest that Paranthropus boisei and P. robustus fed on “low-energy food that had to be processed in great quantities”, “a hard object diet”, “food objects... hard and round in shape” (Demes & Creel, 1988). According to studies on molar enamel microwear of South African australopithecines, “Paranthropus ate substantially more hard food items than Australopithecus” (Grine & Kay, 1988). Studies on incisal microwear suggest that “P. robustus may have ingested foods that required less extensive incisal preparation than the foods consumed by A. africanus” (Ungar & Grine, 1991), but “incisors need not be employed in the manipulation of hard objects” (Ungar & Grine, 1989). However, the precise nature of the robust australopithecine diet is still unknown. A solution may be found in the remarkable parallelism between the dentitions of robust australopithecines, especially P. boisei, and the giant panda, Ailuropoda melanoleuca. In comparison with respectively non-robust australopithecines and non-panda bears, both have less prognathic faces, relatively smaller incisors and canine teeth, broader and heavier cheek bones, broader molars and premolars and “molarized” premolars, and thicker molar and premolar enamel (Aiello & Dean, 1990; Du Brul, 1977; Grassé, 1955). The heavy grinding apparatus of P. boisei could have been an adaptation for processing, among other things, tough parts of bamboo plants, on which giant pandas almost exclusively feed. The stalks of bamboo and other Gramineae such as sugar cane fit the description of low-energy food as well as that of hard and round food objects. P. boisei has been discovered in former lagoons (Carney et al., 1971) and montane forests (Bonnefille, 1976), and P. robustus, near streamside or marsh vegetations (Brain, 1981, p. 189). In such environments the bamboo or reed species on which some primates feed are abundant (e.g. MacKinnon, 1978; Glander et al., 1989). This diet is not as unlikely for a hominid as it may seem. Humans eat grains of different Gramineae (rice, com, wheat), and our closest relatives are known to feed also on harder parts of Gramineae: common chimpanzees like to chew sugar cane stalks, and young mountain gorillas love the young shoots of bamboo while the adult males crack the stalks of bamboo (MacKinnon, 1978). Other primates that cat different parts of bamboo are Rhinopithecus roxellana, Cercopithecus mitus kanditi, Callicebus moloch and three Hapalemur species (Glander et al., 1989). An electron microscope study of the enamel surface of the teeth of Gigantopithecus blacki indicates that also this fossil ape, which developed thick enamel and strongly molarized premolars in parallel with the robust australopithecines, fed partly on Gramineae, possibly bamboo (Ciochon et al., 1990). It must be possible to test this hypothesis by comparing molar enamel microwear of Gigantopithecus, Paranthropus and Ailuropoda. References Aiello L. & Dean C., 1990. Human Evolutionary Anatomy, Academic Press, London. Bonnefille R., 1976. Implications of pollen assemblage from Koobi Fora Formation, East Rudolf Kenya. Nature, 264: 403-407. Brain C. K., 1981. The hunters or the hunted? University of Chicago Press, Chicago. Carney J., Hill A., Miller J. A. & Walker A., 1971. Late australopithecine from Baringo District, Kenya. Nature 230: 509-514. Ciochon R. L., Piperno D. & Thompson R. G., 1990. Opal phytoliths on the teeth of the extinct ape Gigantopithecus blacki: implications for paleodietary studies. Proceedings of the National Academy of Sciences USA, 87: 8120-8124. Demes B. & Creel N., 1988. Bite force, diet, and cranial morphology of fossil hominids. Journal of Human Evolution, 17: 657-670. Du Brul E. L., 1977. Early hominid feeding mechanisms. American Journal of Physical Anthropology, 47: 305- 320. Glander K. E., Wright P. C., Seigler D. S., Randrianasolo V. & Randrianasolo B., 1989. Consumption of cyanogenic bamboo by a newly discovered species of bamboo lemur. American Journal of Primatology, 19: 119-124. Grassé P. P., 1955. Traité de Zoologie, Masson & Cie, Paris, Vol. 1 7. Grine F. E. & Kay R. F., 1988. Early hominid diets from quantitative image analysis of dental microwear. Nature, 333: 765-768. MacKinnon J. R., 1978. The ape within us, Collins, London. Ungar P. S. & Grine F. E., 1989. Maxillary central incisor wear in Australopithecus and Paranthropus. American Journal of Physical Anthropology, 78: 317. Ungar P. S. & Grine F. E., 1991. Incisor size and wear in Australopithecus africanus and Paranthropus robustus. Journal of Human Evolution, 20: 313-340. AFRICAN APE ANCESTRY Human Evolution 5: 295-297, 1990 It is commonly believed that the australopithecines are more closely related to humans than to African apes. This view is hardly compatible with the biomolecular data, which place the Homo/Pan split at the beginning of the australopithecine period. Nothing in the fossil hominid morphology precludes the possibility that some australopithecines were ancestral to gorillas or chimpanzees and others to humans. Key words: Hominid evolution, gorilla, chimpanzee, Australopithecus, Lucy, Taung. It is commonly thought that from a period covering at least the last four million years, no fossils of ancestors of the African apes have been found so far, although hundreds of hominid fossils have been discovered from that period. The usual explanation for this remarkable absence of fossil apes is low fossilisation probability in tropical forests (where the ancestral apes presumably lived). A more likely solution is that not only man, but also the African apes have descended from the australopithecines (e.g., Gribbin & Cherfas, 1983; Hasegawa et al., 1985; Edelstein, 1987). The molecular clock leaves little doubt that the man/chimp split occurred between 6 and 4 Myr BP (Hasegawa et al., 1985), which is in the beginning of the australopith period from about 6 (Lukeino, Lothagam) until 1 Myr BP (Taung). Australopithecines are generally believed to be closer to man than to apes because of their dental and locomotor features. Like man, they have much thicker molar enamel than apes, but enamel thickness has been secondarily reduced in the African apes (Martin, 1987). The robust forms show much smaller anterior teeth than the adult males of G. gorilla and P. troglodytes (differences with the females are less). But bonobos have rather small and only slightly dimorphic canine teeth (Zihlman et al., 1978). Since the prognathism of Negroes compared with other humans developed in about 200,000 years (Cann et al., 1987), the evolution of the (indeed much more pronounced) ape prognathism in 1 Myr cannot be considered impossible. The humanlike orientations of afarensis, distal femoral and tibial articulations (Stern & Susman, 1983), the short iliac bones of Lucy and A. africanus (McHenry, 1982), and the more central foramen magnum in the robust australopiths and Taung are thought to be correlated with bipedality. However, Gribbin & Cherfas (1983), Hasegawa et al. (1985) and Edelstein (1987) have argued that the African apes’ ancestors were more bipedal. Also bonobos have a more central foramen (Kimbel et al., 1984) and frequently walk bipedally (Zihlman et al., 1978). The mistake of many palaeoanthropologists - the anthropocentric fallacy using «primitive» for «gorilla-» or «chimp-like» - is described by Hasegawa et al. (1985): «It seems to have been widely assumed implicitly that the common ancestor (of man and chimp) was more like the chimpanzee». Cranial resemblances between australopithecines and apes are listed in Table 1. Also «the Homo like features of Australopithecine limb bones tend to have been greatly exaggerated in the literature (O. J. Lewis, pers. comm.). Most afarensis postcranials (AL 288, 129, 333) are different from both humans and apes, but the scapula, humerus, ulna, knee, hand and foot bones are more like apes (McHenry, 1982; Stern & Susman, 1983; Senut, 1981; Feldesman, 1982; Tardieu, 1986; Sarmiento, 1987; Deloison, 1985). Lucy’s pelvic girdle AL 288 resembles the apes in some respects (lateral enlargement of iliac blades, small auricular and acetabular articulation surfaces, small lumbosacral angle; McHenry, 1982; Stern & Susman, 1983; Abitbol, 1987), and her upper limb looks rather bonobo-like (Stern & Susman, 1983; Feldesman, 1982). Also A. africanus scapula Sts 7 (McHenry, 1982), its hand bones (TM 1526) and those of A. robustus (SKW 14147, SK 84 and 85) are more chimp than humanlike (Lewis, 1977). The enormous L40-19 ulna of A. boisei is of gorilla size, and morphologically intermediate between man and common chimp (Feldesman, 1982). Although the picture is confused by the retention of ancestral characters in populations that split not very long before (e.g., large and small A. afarensis) and by parallel evolution (both robust forms lived at the same time), it gives me the following impressions. A. boisei and perhaps some of the larger A. afarensis are closer to Gorilla, while Lucy and the South African australopiths show more affinities with Homo-Pan (but A. robustus, living at the time of KNM-ER 1470, could not belong to the Homo lineage). The Taung child, which lived even later than A. robustus, is perhaps ancestral to Pan paniscus or to Pan troglodytes. Table l - Cranial resemblances of australopiths with apes The australopith dentition is more apelike in development pattern (Conroy & Vannier, 1987), enamel growth rate (Bromage & Dean, 1985), dental morphology (Johanson & Edey, 1981), and enamel microwear. All australopithecine brain endocasts appear to be ape rather than humanlike in size and sulcal pattern (Falk, 1985). The composite A. afarensis skull (mostly AL 333; Kimbel et al., 1984) «looked very much like a small female gorilla» (Johanson & Edey, 1981). The extensive pneumatization of the AL 333-45 temporal bone is also seen in chimpanzee males and some gorillas; «the pattern of pneumatization in A. afarensis is also found only in the extant apes among other hominoids» (Kimbel et al., 1984). KNM-WT 17000 had «extremely convex inferolateral margins of the orbits such as found in some gorillas» (Walker et al., 1986). «The ‘keystone’ nasal bone arrangement suggested as a derived pattern diagnostic of Paranthropus is found in an appreciable number of pongids, particularly clearly in some chimpanzees» (Eckhardt, 1987). A. robustus incus SK 848 resembles Pan more than Homo and certainly than Gorilla (Fig. 1 in Rak & Clarke, 1979). A. africanus Sts 5 resembles a bonobo skull (Zihlman et al., 1978). The Taung skull has much more chimp than human traits (Bromage, 1985) and is indeed much too recent (Partridge, 1985) to be on the line to Homo. Its «pneumatization has also extended into the zygoma and hard palate. This is intriguing because an intrapalatal extension of the maxillary sinus has only been reported in chimpanzees and robust australopithecines among higher primates» (Conroy & Vannier, 1987). References Abitbol M. M., 1987. Evolution of the lumbosacral angle. American Journal of Physical Anthropology, 72: 361-372. Bromage T. G., 1985. Taung facial remodeling: a growth and development study. In: P. V. Tobias, ed. Hominid Evolution, pp. 239-245, Liss, New York. Bromage T. G. & Dean M. C., 1985. Re-evaluation of age at death of immature fossil hominids. Nature, 317: 525-527. Cann R. L., Stoneking M. & Wilson A. C., 1987. Mitochondrial DNA and human evolution. Nature, 325: 31-36. Conroy G. C. & Vannier M.W., 1987. Dental development of toe Taung skull from computed tomography. Nature, 329: 625-627. Deloison Y., 1985. Comparative study of calcanei of primates and Pan-AustralopithecusHomo relationships. In: P. V. Tobias, ed. Hominid Evolution, pp. 143-147, Liss, New York. Eckhardt R.B. Hominoid nasal region polymorphism and its phylogenetic significance. Nature, 328: 333-335. Edelstein S. J., 1987. An alternative paradigm for hominoid evolution. Human Evolution, 2: 169-174. Falk D., 1985. Hadar AL 162-28 endocast as evidence that brain enlargement preceded cortical reorganization in hominid evolution. Nature, 313: 45-47. Feldesman M. R., 1982. Morphometrics of the ulna of some Cenozoic «hominoids». American Journal of Anthropology, 57: 187. Gribbin J. & Cherfas J., 1983. The monkey puzzle, Triad, Paladin. Hasegawa M., Kishino H, & Yano T., 1985. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. Journal of Molecular Evolution, 22: 160-174. Johanson D. C. & Edey M. A., 1981. Lucy, Granada, London. Kimbel W. H., White T. D. & Johanson D. C., 1984. Cranial morphology of Australopithecus afarensis: a comparative study based on a composite reconstruction of toe adult skull. American Journal of Physical Anthropology, 64: 337-388. Lewis O. J., 1977. Joint remodelling and the evolution of the human hand. Journal of Anatomy, 123: 157-201. Martin L., 1987. Significance of enamel thickness in hominoid evolution. Nature, 314: 260263. McHenry H. M., 1982. The first bipeds: a comparison of the A. afarensis and A. africanus postcranium and implications for toe evolution of bipedalism. Journal of Human Evolution, 15: 177-191. Partridge T. C., 1985. Spring flow and tufa accretion at Taung. In: P. V. Tobias, ed. Hominid Evolution, pp. 171-187, Liss, New York. Rak Y. & Clarke R. J., 1979. Ear ossicle of Australopithecus robustus. Nature, 279: 62-63. Sarmiento E. E., 1987. Long bone torsions of the lower limb and its bearing upon the locomotor behavior of australopithecines. American journal of Physical Anthropology, 72: 250-251. Senut B., 1981. Humeral outlines in some hominoid primates and in Pliopleistocene hominids. American journal of Physical Anthropology, 56: 257-283. Stern J. T. & Susman R. L., 1983. The locomotor anatomy of Australopithecus afarensis. American Journal of Physical Anthropology, 60: 279-317. Tardieu C., 1986. The knee joint in three hominid primates: application to Plio-Pleistocene hominids and evolutionary implications. In: D. M. Taub & F. A. King, eds. Current Perspectives in primate Biology, pp. 182-192, Van Nostrand Reinhold, New York. Walker A., Leakey R. E., Harris J. M. & Brown F. H., 1986. 2.5-Myr Australopithecus boisei from west of Lake Turkana, Kenya. Nature, 322: 517-522. Zihlman A.L., Cronin J. E., Cramer D. L. & Sarich V. M., 1978. Pygmy chimpanzee as a possible prototype for the common ancestor of humans, chimpanzees and gorillas. Nature, 275: 744-746. LETTER TO THE EDITOR Human Evolution 2: 381, 1987 Sir, The aquatic ape theory states that our hominid ancestors spent a considerable part of their day swimming end diving in a river, lake or sea, and, at least partially, are aquatic food. The AAT is supported by our lack of body hair, our thick fat-layer and several other features absent in nonhuman primates, but widespread among aquatic mammals (HARDY, 1960; MORGAN, 1982; VERHAEGEN, 1985). The ability to speak is a uniquely human character. Innumerable attempts explaining it have been made, but the question how language emerged has not yet been solved. Recently it has been suggested that the origin of speech was facilitated by our aquatic past (MORGAN, 1982, pp. 92-105; MORGAN & VERHAEGEN, 1986). All aquatic mammals control their breathing «voluntarily», i.e. through the primary motor cortex. When surfaced they open the airway whenever they went to inhale air, and they can hyperventilate and then close the airway whenever they intend to dive. The human primary motor cortex (area 4) is much larger than that of apes, mostly due to the expansion of the areas for the musculature of mouth, throat end breathing. Just in front of that enlarged area 4 lies the typically human Broca’s area. In present-day man, it coordinates the activities of the enlarged area 4, to produce the right sound on the right time. Brcoca’s area may have been originated in a previous aquatic phase to coordinate the muscles commanded by the enlarged area 4, to make the right airway muscle contract on the right time: just before, during or just after a dive. In order to use this voluntary control for improving his vocalizations, our ancestor must have been able to interpret his own sound production (feedback). This was improved by the evolution of the arcuate fasciculus, a typically human pathway between Broca’s area end Wernicke’s area (GESCHWIND, 1972). Wernicke’s area, a primary language area used for decoding spoken language, lies dorsal to primary auditory cortex end to the principal sensory areas for mouth end throat. In Wernicke’s area, connections could be made with other nearby association trees, and a certain sound or combination of sounds could be associated with something that our ancestor was aware of (hearing, seeing, feeling, doing) at the same time. Compared with a chimp’s brain our association areas are enormously enlarged. These areas amplified the possibilities of the sound producing apparatus: they act as the hardware of the computer, whereas the sound analysing end producing areas act as the input/output apparatus; the particular language is the software. Most authors discussing language origins try to explain our speech capacity by an enormous improvement of vocalizing abilities that already existed in rudimentary form in prehuman primates, but fail to explain how exactly this could have occurred. In my opinion, most of these problems are readily solved by the application of the aquatic theory to the vocal and breathing apparatus. GESCHWIND N., 1972. Language and the brain. Scient. Amer., 226: 76-83. HARDY A. C., 1960. Was man more aquatic in the past? New Scient., 7: 642-5. MORGAN E., 1982. The aquatic ape. London: Souvenir. MORGAN E. & VERHAEGEN M., 1986. In the beginning was the water. New Scient., 1498: 6263. VERHAEGEN M., 1985. The aquatic ape theory: evidence and a possible scenario. Med. Hypoth., 16: 16-32.