UpInSmoke13
advertisement

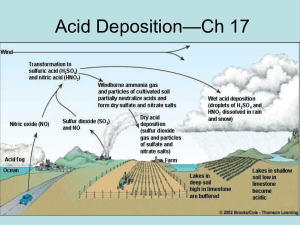
Up in Smoke, Down in pH OBJECTIVES The participant will be able to: 1. Observe the effect of sulfur dioxide and nitrogen oxides on the pH of water. 2. Explain how acid rain affects lakes and streams. BACKGROUND INFORMATION Acid deposition is a serious environmental concern for industrialized nations, most of which are in western Europe and North America. The process of acid deposition begins with emissions of sulfur dioxide (primarily from coal-burning power plants) and nitrogen oxides (primarily from motor vehicles and coal-burning power plants). These pollutants interact with sunlight and water vapor in the atmosphere to form acidic compounds. When either sulfuric acid or nitric acid returns to earth in the form of precipitation, it is called “wet deposition” or “acid rain.” When acid-forming dry particles fall to the earth, it is called “dry deposition.” Moving air mass patterns can carry acid forming pollutants from one region to another and from one country to another. Whenever large-scale sources of acidic emissions are located in the paths of prevailing winds blowing toward international borders, there is a potential of acidic damage to another country. For example, concern has been expressed that Mexico’s copper smelter at Nacozari could cause acid rain on the peaks of the Rocky Mountains. Canada and the United States contribute to acid deposition problems in both countries. However, now the United States sends approximately three times as much sulfur dioxide to eastern Canada as Canada sends to the United States because of prevailing wind patterns and the greater quantities of U.S. emissions. The extent of damage caused by acid deposition also depends on the sensitivity of the area receiving it. One factor is the geology of the area. In areas where the bedrock is limestone, the acidity can be neutralized to some degree by acid-base reactions; limestone is basic (alkaline) and can react with the acid, lessening its impact. In mountainous areas, the bedrock is frequently granite, which does not neutralize acid. In such areas, acid deposition enters surface and groundwater systems virtually un-neutralized, causing the acidity of these systems to increase and affecting sensitive plants and animals. The pH is a measure of the number of hydrogen ions present in a solution. It is expressed on a scale of 0 to 14, where 7 is neutral. The concentration of positively charged hydrogen ions determines whether a solution is acidic or basic (alkaline). More hydrogen ions in a solution make it more acidic; fewer hydrogen ions in a solution make it less acidic (or more alkaline). Values below a pH of 7 indicate acidity, and values above 7 indicate basicity (alkalinity). The pH scale is a logarithmic scale. A one-unit change on the pH scale represents a ten-fold change in acidity. For example, a change from pH of 7 to 4 represents a l0 x l0 x 10 or a l,000-fold change in acidity. The normal pH of rainwater is between 5 and 6. Any rainwater with a pH below that of normal rainwater is considered acid rain. Revised 12/27/13 PROCEDURE A. Sulfur dioxide (SO2) and nitrogen oxides (NOX) for this experiment will be generated by combining two compounds — liquid sulfuric acid and solid sodium nitrite. CAUTION: Safety glasses should be worn by all participants during the experiment. Make sure the room has adequate ventilation. 1. Set up the apparatus as shown in the following diagram. sulfuric acid + sodium nitrite 100 ml water 2. Pour 100 milliliters (ml) of room-temperature, distilled water into a beaker. Measure the pH of the distilled water and record it in the data table. 3. Place 2.0 grams of sodium nitrite in the reaction flask. 4. Place one end of the glass tube into the beaker of water. IMPORTANT: The glass tube must remain under the surface of the water until the reaction is complete. 5. Wear safety glasses! Use a graduated cylinder to measure 10 ml of 5-percent sulfuric acid. Now pour the 10 ml of 5-percent sulfuric acid into the reaction flask containing the sodium nitrite. Immediately seal the flask with the stopper containing the glass tube. Gas should begin to bubble at once through the 100 ml of water. Gentle shaking of the reaction flask may be required to completely react all of the sodium nitrite. Approximate reaction time is 5 minutes. 6. After the gas has stopped bubbling through the water, check the pH of the 100 ml of water and record it in the data table. 7. Clean up by flushing the graduated cylinder and beaker with water. The instructor will clean the reaction flask. Revised 12/27/13 B. Compare the pH of the 100 ml of water before the SO2 and NOX passed through it to the pH after the gases were introduced into the water. 1. Record the total number of pH units the water changed during the experiment. 2. Estimate how many times more acidic the water is after the reaction with SO2 and NOX (REMINDER: Every unit of change corresponds to a change in the acidity by 10 times. A 1-unit decrease in pH would be 10 times more acidic; a 2-unit change would be 10 x 10 or 100 times more acidic.) ___________________________________________________________________________ ___________________________ Name Data for Up in Smoke, Down in pH pH of Distilled Water pH of Distilled Water + SO2 + NOX Change in pH How many times more acidic is the water after the reaction with SO2 and NOX ? What might happen in a lake or stream that undergoes a similar pH change? In what part(s) of the experiment did you have to be especially careful? Why? __________________________________________________________________________ CREDIT: This activity was adapted from the Air and Waste Management Association Environmental Resource Guide – Air Quality. Revised 12/27/13