HERE - Lnk2Lrn
advertisement

Name ______________________________________ IB Physics: Thermal Energy. Show all work to get credit. 1. Complete the following statement: A temperature decrease of 30 C° is equal to a temperature decrease of (a) 30 F° (c) 17 F° (e) 303 K. (b) 30 K (d) 26 F° 2. Absolute zero on the Celsius temperature scale is –273.15 °C. What is absolute zero on the Fahrenheit temperature scale? (a) –331.67 °F (c) –395.67 °F (e) –459.67 °F (b) –363.67 °F (d) –427.67 °F 3. The digital sign outside a local bank reports that the temperature is 44 °C. What is the temperature in degrees Fahrenheit? (a) 56 °F (c) 99 °F (e) 120 °F (b) 79 °F (d) 111 °F 4. How much heat is liberated when 525 kg of Iron cools from 850° C to 37° C? The specific heat capacity of Iron is 450 J / kg°C. (a) 2.5x109 J (c) 1.9x108 J (e) 8.5x109 J (b) 2.9x108 J F (d) 2.9x107 J 5. The 4 ways to transfer heat are (a) conduction, convection, respiration, transpiration (b) evaporation, conduction, convection, radiation (c) conduction, exhalation, convection, respiration (d) conduction, exhalation, evaporation, respiration (e) contraction, exhalation, evaporation, expansion 6. Which one of the following statements is the best explanation for the fact that metal pipes that carry water often burst during cold winter months? (a) Water contracts upon freezing while the metal expands at lower temperatures. (b) The metal contracts to a greater extent than the water. (c) The interior of the pipe contracts less than the outside of the pipe. (d) Water expands upon freezing while the metal contracts at lower temperatures. (e) Both the metal and the water expand, but the water expands to a greater extent. 7. The coefficient of linear expansion of steel is 12 × 10–6/C°. A railroad track is made of individual rails of steel 1000 m in length. By what length would these rails change between a cold day when the temperature is –10 °C and a hot day at 30 °C? (a) 0.62 cm (c) 48 cm (e) 620 cm (b) 24 cm (d) 480 cm 8. Complete the following statement: The term heat most accurately describes (a) the internal energy of an object. (b) a measure of how hot an object is. (c) the absolute temperature of an object. (d) the molecular motion inside of an object. (e) the flow of energy due to a temperature difference. 9. The metric unit of heat, the Joule, is also used to measure which one of the following quantities? (a) force/time (c) temperature (e) power (b) energy (d) specific heat capacity 10. A 2.00-kg metal object requires 5.02x103 J of heat to raise its temperature from 20.0 °C to 40.0 °C. What is the specific heat capacity of the metal? (a) 63.0 J/(kg C°) (c) 251 J/(kg C°) (e) 1000 J/(kg C°) (b) 126 J/(kg C°) (d) 502 J/(kg C°) 11. Which would cause a more serious burn: 30 g of steam or 30 g of liquid water, both at 100 °C; and why is this so? (a) Water, because it is denser than steam. (b) Steam, because of its specific heat capacity. (c) Steam, because of its latent (hidden) heat of vaporization. (d) Water, because its specific heat is greater than that of steam. (e) Either one would cause a burn of the same severity since they are both at the same temperature. 12. The two properties of the Kinetic Theory are: (1) All matter is composed of tiny particles (atoms and/or molecules)., and (2) (a) These particles always stick together. (b) These particles are in constant position. (c) These particles are hard to see. (d) These particles are in constant motion. (e) These particles are hot and cold. 13. The 4 States of Matter are: (a) solid, vapor, gas, liquid (c) plasma, vapor, gas, liquid (e) plasma, vapor, gas, liquid (b) solid, heat, gas, liquid (d) solid, liquid, gas, plasma 14. At what temperature does Fahrenheit equal Celsius? (a) 100 (b) 32 (c) -40 (d) -100 (e) 80 15. Kinetic Theory tells us that (A) heat spontaneously flow from a hot to a cold object. (B) all matter is composed of tiny particles. (C) particles that make-up all matter are constantly moving. (D) Both A and B. (E) Both B and C. 16. What is the efficiency of a heat engine if a quantity of heat is added at a temperature of 240.0oC and the temperature of the exhaust gases is 90.0oC? (A) 10.8 % (B) 70.8% (C) 29.2 % (D) not given 17. The ___ Law of Thermodynamics is a re-statement of the Law of Conservation of Energy. (A) Zeroth (B) First (C) Second (D) Third 18. Who discover that 4.18 J of mechanical energy is equivalent to 1.0 calories of heat? (A)Sadi Carnot (B) Nicolaus Otto (C) James Watt (D) James Joule (E) Hero of Alexandria 19. Who invented the first heat engine? (Same choices as #17.) 20. What is it that spontaneously flows from a hot to a cold object? (A) caloric (B) entropy (C) temperature (D) heat 21. Of the 4 ways to transfer energy in the form of heat, which one relies on latent heat? (A) conduction (B) convection (C) radiation (D) evaporation



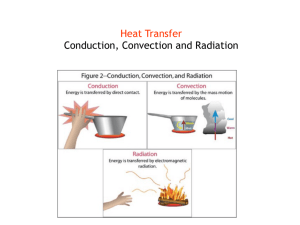



![Applied Heat Transfer [Opens in New Window]](http://s3.studylib.net/store/data/008526779_1-b12564ed87263f3384d65f395321d919-300x300.png)