stereochemistry
advertisement

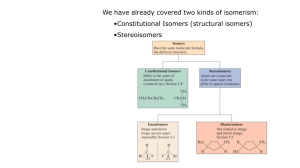
Stereochemistry - - Specific attention has been paid to nomenclature to state the exact spatial relationship between substituents on a carbon – spatial relationship lends different chemical and physical properties to a molecule. The chemistry concerned with the 3D structural arrangement of the atoms in a molecule is called Stereochemistry Stereochemistry and Carbon - think about the relationship between your two hands – they're made up of the same components (thumb and fingers, palm), but they are not identical – they're mirror images of one another. - Mirror images cannot be rotated to be super-imposable upon one another. - Molecules that have the same composition (isomers) but are not super-imposable upon its mirror image are enantiomers. - In our chemistry, results when you have a tetrahedral carbon bound to 4 separate substituents: - - This property is known as chirality: o A chiral molecule forms when the molecule and mirror image cannot be superimposed: they do not contain a plane of symmetry: o Plane of symmetry is a line that transects the object so one half is same as other. o Flask has plane, hand, screw, shoe do not A chiral atom most commonly C with 4 substiturents, and a carbon bound this way is known as a chiral center, or stereocenter. Chirality is a molecular property, a stereocenter is the cause of chirality: Optical Activity - Plane polarized light o Light rotates in an infinite # of planes perpendicular to the direction of travel o when passed through a polarizer, only light of a single plane passes through: plane polarized: example - Example of something of everyday use that is polarized? (sunglasses – vertical slits don't allow horizontal light to pass – therefore glare reduced) HTML page - By rotation of 2 polarizers, we can determine the extent of rotation that has occurred: using a polarizer (1st filter) and an analyzer (2nd filter). - First is – a degree measurement – determines the extent of rotation of the light, looking from observer to analyzer filter to point where light passes through 2nd filter – if the polarizer is turned to the right (clockwise) – dextrorotary (D); to the left (counter clockwise) – levorotary - By convention, Levorotary(L) is negative (-) and Dextrorotary is positive (+) MOVIE Rotation Data - what can tell us? - The extent of rotation depends on more than just the structure of the molecule – it depends upon the number of molecules that the light will interact with – so more molecules either by increasing concentration or increasing the depth of the sample, the larger the rotation ie. Increase concentration 2X, a increases 2X - So set of standard conditions are therefore set by convention : known as the specific rotation []D - []D = observed (rotation)/ l (dm) c (g/mL) = /lc when wavelength of 598.6 nm (sodium line) is used. - This is a physical constant characteristic of the compound Enantiomers - optical isomer - discovered by Pasteur (1849) when noticed 2 types of crystals formed in tartaric acid, from an optically inactive solution; separate the crystals; redissolved; and the 2 resultant solutions were both active - Identical physical properties, different optical properties How do we name chiral centers? - Uses similar set of rules as for E-Z nomenclature 1. priority ot atomic number 2. look at 2nd , 3rd and 4th atoms outward to find first difference 3. multiple bonds equalivalent to same number of single atoms - number them from highest to lowest (1-4) Put bond four into background (into page), and result in a three spoke wheel; place position 1 at top (12 o'clock) If 1 to 2 to 3 turns right, it is R (rectus latin right) If 1 to 2 to 3 turns left, it is S (sinister latin left) (+) and (-) optical rotation and R, S are NOT RELATED Diasteromers - examples shown previously have single chral centers – what happens when you have more than one chiral center? - 2 amino 3 hydroxy butanoic acid (tartaric acid) - results in two paris of enantiomers (2 sets of mirror images): how do we describe the 2 pairs which are not mirror images? These are diasteromers Enantiomers are opposite at ALL stereocenters; Diasteromers are opposite at only some sterocenters What happens is the steroisomer has a plane of symmetery? - not an optically active compound as it is achiral - called meso compounds – sterocenters exist, but are not optically active Biological compounds are specific: Cholesterol: Racemic mixtures - mixture which is a 50:50 mix of both steroisomers. - Denoted by (+/-) or d,l - Most racemic mixtures resolved by generating an acid base reaction to form a salt. - Steroisomers have different properties: Table 6.3 text Relationship between Types of Isomerism: