Pre-lab for The Flaming Cheeto Lab for Pyro's
advertisement

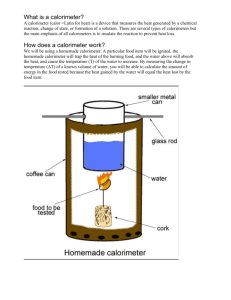
Name__________________________Hour___________Date______________ Calorimetry Lab: You need to know the following: 1. Energy can be measured using a unit called the calorie. Joules are also valid but in this experiment we will use calories. 2. The definition of a calorie is the following: One calorie is the amount of energy required to raise the temperature of one gram of water one degree Celsius. Read that over a few times and learn it. You will need to know it. 3. 1000 calories we measure in lab = 1 Calorie that is on a food label. Did you notice the “calorie” label became a capitol letter? 1. First, we need some food with lots of energy in it. Which one of these foods would you guess has the most energy? potatoes pasta peanuts peas pork pineapple 2. Why? 3. A calorie is the amount of _________needed to raise the __________of ______one degree 4. What is the density of water? 5. What would the mass be of 20 mL of water? Show work. 6. What would the mass be of 30 mL of water? Show work. 7. What would the mass be of 40 mL of water? 8. Is there a pattern here? Why? Name__________________________Hour___________Date______________ Describe the experiment that you are about to do as your teacher explains it to you. Pre-lab Questions 1. Where does the energy stored in a peanut originally come from? 2. During what process was this energy stored in the nut, & where specifically was it stored? 3. What simple sugar made by plants is a common source for stored energy? Name__________________________Hour___________Date______________ 4. Which group of macromolecules would a nut contain --- carbohydrates, lipids, or protein? 5. A generalization is that carbohydrates provide about 4 Cal/g while fat provides about 9 Cal/g. (1 Cal = 1 dietary calorie). How would you expect a peanut to compare to a cheeto? 6. What is the name for stored energy? 7. Give some examples of how organisms would use this stored energy. 8. What is the purpose of this experiment? 9. In this experiment, what will happen to the energy stored in the nut? Where will it end up? Draw a flow chart of how it is transferred. 10. What is the law of Conservation of mass? 11.Would you expect the final mass of the nut to be less than the original mass of the nut? 11. Write the calorimetry equation out in the space below and then complete the key for each variable complete with labels. Name__________________________Hour___________Date______________ Key ______= ______= ______= ______= 12. You want to raise the temperature of 250 mL of water from 22 degrees Celcius to 99 degrees Celcius. How much thermal energy will be needed? 13. How much thermal energy will be needed to raise 4 times the amount of water (instead of 250 mL----1000 mL)? Name__________________________Hour___________Date______________ 12.ACTIVITY: PEANUT CALORIMETRY LAB The evening before a marathon, runners are advised to eat a huge plate of pasta. Why? Because pasta, a carbohydrate, is a terrific source of energy, or fuel for the body. Different foods contain varying amounts of energy that is stored in the bonds of the chemicals they are composed of. Proteins, carbohydrates and fats all have different bonds between different elements that can be broken to release energy. Your mitochondria in your animal cells can convert this energy into ATP energy and other energy storing molecules to utilize later when you need it. This process of converting food energy into usable energy for your body is called cellular respiration. In this activity, you will demonstrate how calories are measured. First, you need to build an inexpensive calorimeter. A calorimeter is a device that measures the transfer of heat energy during a chemical or physical change. One type of calorimeter contains a combustion chamber surrounded by water. When matter is placed in the chamber and physically or chemically changed (usually by burning), the temperature change of the surrounding water is measured and used to determine the energy (calorie) content of the sample. This lab indirectly measures food Calories (kilocalories) using a homemade calorimeter. MATERIALS: goggles ring stand wire ring large cork matches balance water 200ml beaker 12-oz. soda can Celsius thermometer compass or scissors gloves or forceps shelled peanut or other high-fat food such as a potato chip Name__________________________Hour___________Date______________ CAUTION: Be sure the room is well-ventilated. Use gloves or forceps to handle hot equipment and burned peanut. PROCEDURE 1. Punch four holes evenly around a soda can, 2 to 3cm from the top (use a compass or scissors). Place two straightened paper clips (or pieces of a wire hanger) into opposing holes. Hang the can in the wire ring on the ring stand and adjust the height accordingly. Add 200ml of water to the soda can. This apparatus is your calorimeter. 2. Straighten part of the third paper clip and leave one end bent. Turn the cork upside down and poke the straight end into the cork. The bent or looped end of the clip (it should be flat) is the platform for the peanut. 3. Place the thermometer into the mouth of the can. Remember to suspend the thermometer in the water when taking a temperature reading. Before going any further, check the apparatus to make sure that everything is secured. 4. Choose a peanut, measure its initial mass and record in the data table (below). 5. Take an initial temperature reading of the water in the can and record in the data table. 6. Place the peanut on its stand. Use a match to set the peanut on fire. This may take several tries. Closely observe the nut as it burns. If the peanut falls off the stand, start over immediately. 7. As soon as the peanut stops burning, immediately take a final water temperature reading and record it in the data table. 8. As soon as the peanut has cooled, use forceps to lift the burned remains onto the balance. Take a final peanut mass and record in the data table. 1. ACTIVITY EXTENSIONS Discuss the sources of heat loss during this experiment. Can you suggest a better design? Discuss the pros and cons of eating peanuts as a staple in the average American diet. Create a diet for an athlete, taking into consideration metabolism of fats, proteins and carbohydrates. Name__________________________Hour___________Date______________ Note: Expect the kcal for the peanut to range between 2 and 8. This activity is an adaptation of a classic calorimeter lab. The idea for using a soda can originally appeared in Science Teacher, September 1992 (p. 54), contributed by Peter Markow of Saint Joseph College in West Hartford, Connecticut. The activity has been further adapted and is frequently used by chemistry teacher Amy Fradkin. DATA Sample 1 Sample 2 Food used _______ _______ Mass of food and holder (initial) ______ g ______ g Mass of food and holder (final) ______ g ______ g Mass of empty can ______ g ______ g Mass of can plus water ______ g ______ g Initial water temperature ______ °C ______ °C Final water temperature ______ °C ______ °C PROCESSING THE DATA 1. Calculate change in water temperature, t, for each sample, by subtracting the initial temperature from the final temperature (t = tfinal – tinitial). 2. Calculate the mass (in g) of the water heated for each sample. Subtract the mass of the empty can from the mass of the can plus water. 3. Use the results of Steps 1 and 2 to determine the heat energy gained by the water (in J). Use the equation Q = t•m•cp where Q = heat absorbed (in J), t = change in temperature (in °C), m = mass of the water heated (in g), and cp = specific heat capacity (4.185 J/g°C for water). Name__________________________Hour___________Date______________ 4. Calculate the mass (in g) of each food sample burned. Subtract the final mass from the initial mass. 5. Use the results of Steps 3 and 4 to calculate the energy content (in J/g) of each food sample. 6. Record your results and the results of other groups below. Share data with other groups. Class Results Food Type Food Type Food Type Food Type ____________ ____________ ____________ ____________ _________ J/g _________ J/g _________ J/g _________ _________ J/g _________ J/g _________ J/g _________ _________ J/g _________ J/g _________ J/g _________ _________ J/g _________ J/g _________ J/g _________ _________ J/g _________ J/g _________ J/g _________ _________ J/g _________ J/g _________ J/g _________ Avg. _________ J/g _________ J/g _________ J/g _________ J/g J/g J/g J/g J/g J/g J/g 7. Which of the foods has the greatest energy content? Why do these foods have the greatest energy content? Name__________________________Hour___________Date______________ Energy Content Information The lab can be performed with food or different fuels such as ethyl alcohol, lamp oil, or candles (paraffin wax). See Heats of Combustion for an example of using the candle as a source. Suggestions and possible results: 1. Alcohol burners may be used but be aware of the danger in their use. Due to the rapid evaporation of the alcohol the mass of the alcohol and burner should be determined immediately before and after use. 2. Lamp oil can be used in alcohol burners. 3. Aluminum drink cans work well. 4. Have ice water available and make sure all the ice is removed before use. Water initially at 4-5 C gives best results, because starting 17-19 below and finishing 1719 C above room temperature tends to equalize heat exchange with the room. 5. Make sure candles are mounted on cardboard bases. The base will catch the drippings. Use 100 mL of water for candles and 200 mL of water for alcohol and oil. 6. Typical class averages: candle 23 000 – 25 000 J/g lamp oil 16 000 – 18 000 J/g ethyl alcohol 12 000 – 14 000 J/g 7. Actual values are about 42.0 kJ/g for paraffin wax and 26.8 kJ/g for ethyl alcohol. You may want to discuss factors causing the results to be lower than actual values. 8. You may want to look at other considerations such as convenience, cost, availability, burning efficiency, and renewability. 9. If you are doing the energy content of food, use a paper clip and small jar lid (baby food jar). Partly straighten the paper clip, bending a small loop in one end. This will cradle the food sample. Glue the other end into the lid which will help catch burned food that might fall. 10. Good choices are cashews, marshmallows, peanuts, and popped popcorn. Because peanuts and cashews release very large amounts of heat you may want to use 100 mL of water in the can and only 50 mL for the others. Name__________________________Hour___________Date______________ 11. You can substitute calories for the heat energy unit. One Calorie = 1000 calories. 12. Typical class averages: Cashews 11 000 – 12 000 J/g (2.5 – 2.9 Cal/g) Marshmallows 4 200 - 5 800 J/g (1.0 – 1.4 Cal/g) Peanuts 11 000 – 12 500 J/g (2.6 – 3.0 Cal/g) Popcorn 5 000 - 8 400 J/g (1.2 – 2.0 Cal/g) 13. Cashews and peanuts have the highest energy content. A generalization is that carbohydrates provide about 4 Cal/g (unbuttered popcorn and marshmallows) while fat rich peanuts and cashews provide about 9 Ca/g. (1 Cal = 1 dietary calorie). The Energy Content of Foods lab and data from this page from “Physical Science with Computers” of Vernier Software, Portland Oregon.