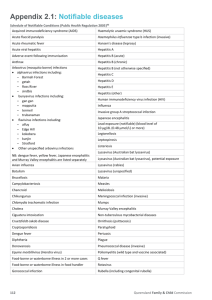
Emerging Diseases, Bacterial Pathogenesis & Antibiotics
advertisement

EMERGING AND REEMERGING DISEASES Dr. Egidia Miftode Table 1. Emerging infectious diseases Emerging infections Bartonella henselae Ehrlichia caffeensis/equi Fakeeh virus Hepatitis G virus Human herpesvirus-8 Andes virus, Bayou virus Laguna negra virus, New York virus, Oran virus Nipah virus Helicobacter pilory Nontuberculous mycobacteria Prion protein Cryptosporidium, Cyclospora, Microsporidia Associated diseases Cat-scratch disease Human monocytic/granulocytic ehrlichiosis Haemorrhagic fever in Saudi Arabia Uncertain significance Kaposi’s sarcoma Hantavirus pulmonary syndrome in South/North America Encephalitis Gastritis/ulcer Infections in HIV patients New variant Crewtzfeld-Jacob disease Gastroenteritis Table 2. Resurgent infectious diseases Disease agent Borrelia burgdorferi Dengue virus Ebola Echerichia coli O157:H7 Japanese encephalitis Leishmania donovani Machupo virus Marburg virus Rift valley fever virus Toscana virus Yersinia pestis Associated disease Lyme disease Dengue haemorrhagic fever/shock Haemorrhagic fever in Gabon/Congo Gastroenteritis/haemolytic uraemic syndrome Encephalitis in Nepal Visceral leishmaniasis Haemorrhagic fever Haemorrhagic fever Haemorrhagic fever Encephalitis in Italy Plague Infectious diseases will continue to emerge, reemerge and submerge, but continuing advances in diagnostics, surveillance, therapeutics and vaccines may be very important in prevention and control. Finally, we must be prepared for the unpredictable and unexpected, as was the case with the emergence of AIDS. PATHOGENESIS OF BACTERIAL INFECTION Egidia Miftode The virulence of pathogen The fitness of a pathogen can be defined as its ability to multiply within a host, disseminate from that host, translocate to a new host, colonize the new host, and cause infection. This can be distinguished from the virulence from a pathogen, which refers to the severity of clinical illness resulting from infection. Characteristics of bacteria that are pathogens include: 1. Transmissibility: from animal to humans/from human to human By food products to humans: Salmonella, Campylobacter By the fleas to humans: Yersinia pestis By animal products(raw hair): bacillus anthracis By ingestion: C. botulinum When wounds are contaminated by soil:C. perfringens and C. tetani. From one person to another by hands: S. aureus The most frequent portal of entry of pathogenic bacteria are: - respiratory - gastrointestinal - genital - urinary tracts 2. Adherence to host cells Once in the body bacteria must attach to host cells, usually epithelial cells They multiply and spread directly through tissues or via the lymphatic system to the blood stream Adherence factors: Surface hydrophobicity and net surface charge (the more hydrophobic the bacterial cell surface, the greater the adherence to the cell surface Specific surface molecules -pili( eg. E. coli) -fimbriae (group A Streptococci); lipoteichoic acid and M protein are found on the fimbriae. 3. Invasion of host cells and tissues This is a term commonly used to describe the entry of bacteria into host cells. Examples: - Shigella adhere to host cells, induces the formation of pseudopods and engulfment of the bacteria. Once inside the cells, the shigellae either are released, or escape from the phagocytic vesicle, where they multiply in the cytoplasm (similar for Yersinia enterocolitica. - Legionella pneumophila - infects pulmonary macrophages and cause pneumonia. The agent induces formation of a thin pseudopod, that coils around the bacteria forming a vesicle. Phagolysosome fusion is inhibited and the bacteria multiply within the vesicle. 4. Toxigenicity Table 1. Characteristics of exotoxins and endotoxins EXOTOXINS ENDOTOXINS Excreted by living cells; high Part of the cell wall of gramconcentrations in liquid medium negative bacteria. May not need to be released to have biologic activity; Produced by both GP and GN Only in gram negative bacteria; bacteria; Polypeptides; Lipopolysacharide complexes; Relatively unstable; toxicity often Relatively stable, without loss of destroyed rapidly by heating(>60 toxicity for hours if >60 C; C); Highly antigenic; stimulate Weakly immunogenic; antibodies formation of antitoxin. Antitoxin are antitoxic and protective; neutralizes toxin; Non converted to toxoids Converted to antigenic, nontoxic toxoids by formalin, acid, heat. Toxoids are used to immunize(tetanus toxoid); Highly toxic(fatal in microgram Moderately toxic; quantities); Usually bind to specific receptors Specific receptors not found in on cells; cells; Usually do not produce fever Produce fever by release of IL1 and other mediators Controled by extrachromosomal Synthesis directed by genes (plasmids) chromosomal genes 5. Ability to evade the host’s immune system Enzymes: coagulase, beta-lactamases, streptokinases METHODOLOGIES USED IN THE DIAGNOSIS OF INFECTIOUS DISEASES Egidia Miftode The methods of diagnosis used for the diagnosis of infectious diseases are as follows: I. II. 1. Serology: requires a diagnostic titer or evidence of conversion by four-folds increase in titer of antibodies: Complement fixation (CF) – Neutralizing antibody titer (NA) -; Hemagglutination inhibition (HI) – Precipitating reaction (when soluble antigen comes in contact with its corresponding antibody in solution, antigen-antibodies complexes result, which are insoluble) -. Immunofluorescence and immunoenzyme procedures – Radioimmunoassay and enzyme immunoassay – Direct detection by: Special stains: Gram stain, acridine orange, mycobacterial stains (ZiehlNeelsen, fluorochrome stain),. 2. Antigen detection by: a. latex agglutination – for CSF specimens, fungal antigens b. enzyme immunoassay (ELISA)– for bacterial antigens (group A sterptococcus, Legionella), bacterial toxins (toxins of E.coli, Clostridium difficile), fungal antigens, parasitic antigens (Giardia lamblia, Cryptosporidium), viral antigens (Herpes simplex, influenza A, adenovirus, respiratory syncitial virus), chlamydial antigens. c. Counter immune electrophoresis (CIE) 3. Molecular based assays: a. Hybridization with DNA probe (detect specific ribosomal DNA) b. Amplification assays such as those using PCR (polymerase chain reaction) or ligase chain reaction. III. Detection by culture and: a. identification by biochemical reactions (ex. coagulase test) b. identification by specific antisera by agglutination or fluorescent antibody test c. identification using molecular-based methods (specific probes, restriction enzyme pattrern, DNA sequencing) antimicrobial susceptibility testing. Specific susceptibility testing procedures a. Disk diffusion susceptibility testing b. Minimal inhibitory concentration testing c. Testing for bactericidal activity d. Serum bactericidal test e. Synergy testing. IV. Skin tests 1. Toxin neutralization 2. Delayed hypersensitivity –. ANTI-INFECTIVE THERAPY Egidia Miftode Antibiotics are bacterial or fungal products, or are obtained by synthesis, which are capable of inhibition or destruction of some microbiological species. The modern era of chemotherapy begins with the discovery of sulphonamides in 1936, followed immediately, in the 1940s, by the discovery of the therapeutic properties of penicillin and streptomycin. Principles of anti-infective therapy Choice of the proper antimicrobial agent 1. Identification of the infecting organism, or, if it is impossible to determine the exact nature of the organism, may be useful to use bacteriologic statistic (application of knowledge of the etiology most likely to cause an infection in a given clinical setting); 2. Determination of antimicrobial susceptibility of etiological agent (sensitive, intermediate sensitive, resistant); 3. Identifying host factors that may influence the efficacy and toxicity of antimicrobial agents: a. History of previous adverse reactions to antimicrobials b. Age (ex. creatinine clearence may be reduced in elderly) c. Genetic or metabolic abnormalities d. Pregnancy Table 1. Antimicrobials in pregnancy Antibiotic Penicilline Cephalosporins Macrolides Polipeptides Tetracyclines Aminoglycosides Phenicoles Rifampicine Sulphonamides Quinolones Nitrofurantoin Imidazoles I-st trimester + + + + - II-nd trimester + + + + + + + III-rd trimester + + + + + + e. Renal and hepatic function (the major routes of drugs elimination) Creatinine clearence (ml/min)=(14—Age in years) x weight in kg/serum Creatinine in mg/dlx72. f. Site of infection.. 4. Antimicrobial combination may be justified in the following situations: When suspected polymicrobial infections (intraperitoneal, pelvic infections, etc) To prevent emergence of resistance (tuberculosis, with antibiotics such as vancomycin, aminoglycosides, fosfomycin) To obtain synergism (ex. penicillin + aminoglycosides for enterococci infections) Initial therapy in neutropenic patients, or, when exact nature of infection is not clear) To decrease toxicity 5. Choice of appropriate route of administration for antimicrobials: - intravenous therapy - oral absorption may be decreased 6. 7. - Pharmacologic properties of antimicrobials. Monitoring the response of the patient to antimicrobial therapy: determination of serum concentration of antimicrobial agents determination of serum bactericidal titer PENICILLINS Classification of penicillins: 1.Natural penicillins 2.Penicillinase resistant penicillins (Methicilline group) 3.Aminopenicillins (“A” Group) 4.Antipseudomonal penicillins (carboxipenicillins and ureidopenicillins) NATURAL PENICILLINS Table 1. Doses of natural penicillins DRUG PENICILLINE G PENICILLINE V EXTENCILLINE (BENZATHINE PEN G) DOSE-ADULT/DAY 1-20 mil.UI, im/iv, 4/6/12h 3-4 mill. U, po , 6/8h 1,2 mill. U/15 days or 2,4 mill.U/month DOSE-CHILD/DAY 50000-200000 U/kg 50000-100000 U/kg 600000 U/15 days or 1,2 mill.U/month Table 2. Antimicrobial spectra and therapeutical indications of natural penicillins ANTIMICROBIAL SPECTRA INDICATIONS 1. Streptococcus group A, B, C, D 2. Str. pneumoniae 3. Str. viridans 4. N. meningitidis 5. C. diphteriae 6. B. anthracis 7. Clostridium (ex. C. difficile) 8. Peptostreptococcus sp 9. Treponema pallidum 10. Leptospira 11.Borrellia 12.Listeria monocytogenes ( )-etiologic agent Tonsillitis, Scarlet fever(1) Pneumonia , meningitis, sepsis (2) Endocarditis(3) Sepsis, meningitis (4) Anthrax (6) Diphteria (5) Syphillis (9) Leptospirosis (10) Lyme disease(11) Meningitis, sepsis (12) PENICILLINASE-RESISTANT PENICILLINS Antibacterial spectra: Staphylococcus aureus (Methicillin-sensitive) Indications: localised / generalised staphylococcal infections Table 3. Doses of penicillinase - resistant penicillins DRUG OXACILLIN / NAFCILLIN CLOXACILLIN DICLOXACILLIN DOSE-ADULT 2-4 g/d (po:6/8h;im,iv:4-12h) 4-12 g/d (im,iv: 4-6h) 1-4 g/d (6-8h) DOSE-CHILD 50-100 mg/day 100-200 mg/kg/day 50-100 mg/kg AMINOPENICILLINS Table 4. Doses of aminopenicillins DRUG AMPICILLIN AMOXICILLIN BACAMPICILLIN METAMPICILLIN PIVAMPICILLIN AMOXICILLIN-clavulanic acid AMPICILLIN-sulbactam DOSE-ADULT Po: 2-4 g/day Im, iv: 4-12 g/day Po,im,iv: 1-8 g/d 0,8-2,4 g/d 2-12 g/d 1-3 g/d 2-3 g/d 3-4,5 g/d DOSE-CHILD 100 mg/d 100-300 mg/d 25-200 mg/kg 50-100 mg/kg/d 50-200 mg/kg/d 50-100 mg/kg/d 30-40 mg/kg/d 30-40 mg/kg/d The antimicrobial spectra and indications: Enterococcus endocarditis Streptococcus gr. A,B,C,G prophylaxis of stomatologic infections S. pneumoniae pneumonia, meningitis, sepsis N. meningitis meningitis, sepsis L. monocytogenes meningitis, sepsis Borrellia meningitis. ANTIPSEUDOMONAL PENICILLINS (CARBOXY AND UREIDOPENICILLINS) Table 5. Doses of antipseudomonal penicillins DRUG TICARCILLINE TICARCILLINE+clavulanic acid MEZLOCILLINE PIPERACILLINE PIPERACILLINE+tazobactam DOSE-ADULT 250 mg/kg/d 12,4-18,6g/d 6-15 g/d 6-15 g/d 4,5-13,5 g/d DOSE-CHILD 75-100 mg/kg/d 150-300mg/kg/d 100-300 mg/kg/d 100-300 mg/kg/d 100-300 mg/kg/d Table 6. Antimicrobial spectra and indications of antipseudomonal penicillins ANTIMICROBIAL SPECTRA OF ANTIPSEUDOMONAL PENICILLINS TICARCILLINE: Proteus Enterobacter Serratia Pseudomonas PIPERACILLINE: “AMPI” spectra + Serratia marcescens Enterobacte cloacae Citrobacter Bacteroides fragilis Pseudomonas Proteus INDICATIONS Severe infections with sensitive germes Plurimicrobial infections: biliary, digestiv, genito – urinary tract MONOBACTAMS AZTREONAM Dose: 1g/day, i.v. in Gram negative cocci and bacilli infections. Adverse reactions: allergies, hepatotoxicity, disulfiram-like syndrome. CARBAPENEMS Table 7. Doses of carbapenems DRUG IMIPENEM MEROPENEM ADULT 2 g/d 3 g/d CHILD 30-60 mg/kg/d 40 mg/kg/d Table 8. Antimicrobial spectra and indications of carbapenems ANTIMICROBIAL SPECTRA *GP COCCI (except Methicillin-Resistant-S. aureus) *GN BACILLI (Ps. aeruginosa, Acinetobacter) *GP ANAEROBES *GN ANAEROBES (Bacillus fragilis) INDICATIONS Severe inf. with multi - resistant germs nosocomial infections Pluribacterial infections GP=gram positive, GN=gram negative CEPHALOSPORINS 1st GENERATION CEPHALOSPORINS Table 9. Doses of 1st generation cephalosporins DRUG CEFACLOR CEFADROXYL CEFALEXINE DOSE-ADULT 2-4 g/d po 2-4 g/d po 2-4 g/d po DOSE-CHILD 50-100 mg/kg/d 50-100 mg/kg/d 50-100 mg/kg/d Table 10. Antimicrobial spectra and indications of 1 st generation cephalosporins ANTIMICROBIAL SPECTRA S. pneumoniae Streptococcus (except enterococcus) Meti-S Staphylococcus INDICATIONS Respiratory infections Urinary infections Skin infections 2nd GENERATION CEPHALOSPORINS Table 11. Doses of 2nd generation cephalosporins DRUG CEFAMANDOLE CEFUROXIME DOSE-ADULT 3-6 g/d 1,5-4 g/d DOSE-CHILD 50-100 mg/kg/d 50mg/kg q8h iv, 10-15mg/kg bid po CEFPROZIL Cephamycines CEFOTETAN CEFOXITIN 500 mg/d, po 10-15mg/kg bid (max 1 g/d) 2-6 g/d, iv 3-6 g/d, iv 30-60 mg/kg/d 50-100 mg/kg/d Table 12. Antimicrobial spectra and indications of 2nd generation cephalosporins ANTIMICROBIAL SPECTRA of 2nd gen. CS 1st gen. cephalosporins – spectra + : H. influenzae N. gonorrhoeae E. coli, Klebsiella For cefoxitine: GN anaerobes INDICATIONS Otitis Sinusitis Tonsilitis (if failure with penicillin G, or reccurences) 3rd GENERATIONS CEPHALOSPORINS Table 13. Doses of 3rd generation cephalosporins DRUG CEFOPERAZONE CEFOTAXIME CEFTAZIDIME CEFTRIAXONE CEFTIZOXIME LATAMOXEF Oral CEFIXIME CEFPODOXIME CEFTIBUTEN CEFDINIR DOSE-ADULT 4 g/d 4-6 g/d 3-6 g/d 2-3 g/d 3-12 g/d 2-6 g/d DOSE-CHILD 30-60 mg/kg/d 50-100 mg/kg/d 50-100 mg/kg/d 50-75 mg/kg/d 30-60 mg/kg q8h 400 mg/d po 200-400 mg/d 400 mg/d 600mg/d 8 mg/kg/d div bid 10mg/kg div bid 4,5 mg/kg bid Table 14. Antimicrobial spectra and indications of 3 rd generation cephalosporins ANTIMICROBIAL SPECTRA of 3rd gen. CFS Enhanced activity vs. AEROBIC GN BACTERIA: E. coli, Shigella, Proteus, Salmonella Inconsistant activity vs.: Serratia, Pseudomonas, Acinetobacter, Enterobacter Modest activity against ANAEROBES (only for CEFTIZOXIME) Antipseudomonal activ.: CEFTAZIDIME Less active than 1st G.CFS. vs. GP COCCI INDICATIONS SEVERE INFECTIONS : Sepsis Broncho-pneum. Nosocomial infections Surgery antibioprophylaxis GN=gram negative; GP=gram positive 4th GENERATION CEPHALOSPORINS CEFEPIME: 1-2 g q12h, i.v. activity vs. Gram positive cocci 3rd Generation CFS CEFPIROME: enhanced activity vs. Enterobacteriaceae and P. aeruginosa. LINCOSAMIDES Table 15. Doses of lincosamides DRUG CLINDAMYCINE LINCOMYCINE DOSE-ADULT DOSE-CHILD 0,6-2,4 g/d, po, im, iv 8-25 mg/kg, po (every 6-8h) 15-40mg/kg, parenterally 0,6-1,8g im., iv po (8h) 30-60 mg/kg/d, po 10-20 mg/kg/d, im/iv THE ANTIMICROBIAL SPECTRA is represented by: Anaerobic bacteria (Gram positive and gram-negative) Staphylococcus Streptococcus (excepting enterococcus) THERAPEUTICAL INDICATIONS are: Bone and joint infections Infections with anaerobes (without meningitis!) Prophylaxis of infective endocarditis (if allergy to Penicillin is present). AMINOGLYCOSIDES Table 16. Doses of aminoglycosides DOSE-ADULT DRUG 1. AMIKACINE 2. DIBEKACINE 3. GENTAMYCINE 4. NETILMYCINE 5. SISOMYCINE 6. TOBRAMYCINE 7. KANAMYCINE 15 mg/kg/d (12-24h) 3 mg/ kg/d (12-24h) 3 mg/kg/d (8, 12, 24h) 4-7 mg/ kg/d (8, 12, 24h) 3 mg/ kg/d (8, 12, 24h) 3-5 mg/ kg/d (8, 12, 24h) 15 mg/kg/d (12h) DOSE-CHILD 15 mg/kg/d 2-4 mg/kg/d 6-9 mg/kg/d 3-5 mg/kg/d 3 mg/kg/d 15mg/kg/d 8. STREPTOMYCINE 9. ISEPAMYCINE 0,5-1 g/d (24h) 15 mg/kg q24h 30-50 mg/kg/d - Table 17. Antimicrobial spectra and indications of aminoglycosides ANTIMICROBIAL SPECTRA of aminoglycosides NETIL.,GENTA.,TOBRA.,SISO.,DIBEKA: - Staphylococcus - E.coli - Listeria monocytogenes Inconstantly: Proteus, Klebsiella, Enterobacter, Serratia AMIKA: - Staphycoccus -Aerobes GN bacilli: E.coli,Serratia Enterobacter, Proteus, Pseudomonas, Klebsiella Acinetobacter STREPTOMYCINE: My. tuberculosis, Brucella KANA: M. tuberculosis INDICATIONS ! IN ASSOCIATION ! -URINARY INFECTIONS. -INFECTIVE ENDOCARDITIS -SEVERE INFECTIONS WITH STAPH/GN BACILLI -INFECTIONS WITH LISTERIA -INFECTIONS WITH MULTIRESISTANT GERMS -BRUCELLOSIS -TUBERCULOSIS TETRACYCLINES Table 18. Doses of tetracyclines DRUG 1st Generation: TETRACYCLINE OXYTETRACYCLINE ROLITETRACYCLINE 2nd Generation: LYMECYCLINE METACYCLINE 3rd Generation: DOXICYCLINE MINOCYCLINE DOSE-ADULT DOSE-CHILD 25-30 mg/kg (6-8h), po 250-500 mg (12-24h),im 300mg,im 50 mg/kg 10-20 mg/kg 10-30 mg/kg (12h) 10-30 mg/kg (12h) 10-15 mg/kg 200 mg (1st day),then 100 mg 200 mg (1st day),then 100 mg 2-4 mg/kg 2-4 mg/kg Table 19. Antimicrobial spectra and indications of tetracyclines ANTIMICROBIAL tetracyclines SPECTRA N. gonorrhoeae, Yersinia Brucella of INDICATIONS Pasteurella Legionella pneumophila Vibrio cholerae Erlichia canis Mycoplasma pneumoniae Rickettsiae Chlamydia Treponema Borrelia BRONCHO-PULMONARY INFECTIONS, BRUCELLOSIS, CHOLERAE, GENITAL INFECTIONS WITH CHLAMYDIA. MACROLIDES Table 20. Doses of macrolides DRUG “old” macrolides: ERYTHROMYCINE SPIRAMYCINE JOSAMYCINE “new” macrolides: CLARITHROMYCINE DIRITHROMYCINE ROXITHROMYCINE Azalides: AZITHROMYCINE SINERGISTINES: PRISTINAMYCINE VIRGINIAMYCINE DOSE-ADULT DOSE-CHILD 2-3 g/d po, iv 2-3 g/d; 4,5-9 mil.UI/d 1-2 g/d 25-50 mg/kg/d 50000 UI/k/d 30-50 mg/k/d 0,5-2 g/d, po 0,5 mg/d, po 300 mg/d 300-600 mg/d 5-8 mg/k/d 500 mg/d, po 10 mg/kg/d 3 g/d, po 3g 50-100 mg/kg 50-100 mg/kg Table 21. Antimicrobial spectra and indications of macrolides ANTIMICROBIAL SPECTRA OF MACROLIDES Streptococcus A,C,G Staphilococcus methicillin-Sensitive Moraxella catarrhalis Bordetella pertussis Corynebacterium diphteriae Erysipelotrix Campylobacter Treponema Legionella Mycoplasma Chlamydia INDICATIONS BRONCHITIS ATYPICAL PNEUMONIA, PNEUMONIA WITH LEGIONELLA, SKIN INFECTIONS, ENTERAL INFECTIONS (YERSINIA, CAMPYLOBACTER, CRYPTOSPORIDIUM), GENITAL INFECTIONS, TOXOPLASMOSIS (pregnancy), BACILLARY ANGIOMATOSIS, Rickettsia GN cocci Toxoplasma gondii ( SPIRAMYCINE, ROXITHRO, CLARITHROMYCINE) Atypical Mycobacteria Bartonella SINERGISTINS: Methicillin-resistant Staphylococcus Penicillin-resistant Pneumococcus GP anaerobes GP=gram-positive; GN=gram-negative HEPATIC PELIOSIS INFECTIONS IN STOMATOLOGY. GLYCOPEPTIDES Table 22. Doses of glycopeptides DRUG VANCOMYCIN ADULT 1,5-2 g iv (12h) TEICOPLANIN 200-400 mg/d CHILD 0-30 days (age): 15 mg/kg/d > 30 days (age): 10 mg/kg/d 10 mg/kg/d The spectra includes: - Methicillin-resistant Stafilococcus aureus (MRSA) - Penicillin-resistant Streptococcus pneumoniae (PRP) - Enterococcus - Clostridium difficile CHLORAMPHENICOL The drug is bacteriostatic for the majority of organisms listed in the following table, but it is bactericid for the germes involved in meningitis: N. meningitidis, S. pneumoniae, H. influenzae. Doses: >28 days old: 12,5-25 mg/kg q6h; older children and adults: 50 mg/kg/day (in 6 hourly intervals); older children and adults with meningitis: 100mg/kg/day (in 6 hourly intervals). Table 23. Antimicrobial spectra and indications of chloramphenicol ANTIMICROBIAL SPECTRA GP cocci: S. aureus S. epidermidis S. pneumoniae Peptococcus-Peptostreptococcus GN cocci and coccobacilli: N. gonorrhoeae Pasteurella multocida H. influenzae Brucella spp Bordetella pertussis GP bacilli: Corynebacterium diphteriae Listeria monocytogenes Clostridium spp Prpionibacterium acnes Actinomyces israelii Actinobacillus actinomycetemcomitans GN bacilli: E. coli, S. typhy, and Proteus mirabilis (the other Enterobacteriaceae have varying sensitivities) Chlamydia, Mycoplasma, rickettsies INDICATIONS BRAIN ABSCESS SUBDURAL EMPIEMA MENINGITIS TYPHOID FEVER RICKETSIAL INFECTIONS QUINUPRISTIN AND DALFOPRISTIN Dose: 7,5 mg/kg i.v. (q 8h) Table 23. Antimicrobial spectra and indications of quinupristin and dalfopristin INDICATIONS ANTIMICROBIAL SPECTRA of Quinupristin and dalfopristin E. faecium Skin infections S. aureus (including MRSA) Hospital aquired pneumoniae S. pneumoniae (including PRP) Urinary tract infections Lactobacillus spp, Bone and joint infections Leukonostoc spp Endocarditis Bacteroides spp Catheter-related bacteremia Moraxella L. monocytogenes Prevotella My. pneumoniae Adverse reactions: local pain, inflammation, phlebitis, gastrointestinal manifestations. METRONIDAZOLE AND OTHER NITROIMIDAZOLES Dose depends upon the infection: Giardiasis: 250 mg bid 5-7 days, or 2g/day 3 days. Susceptible anaerobic infection: i.v. - 15 mg/kg then 7,5 mg/kg q6h p.o. – 1-2 g/d in 2-4 doses . Antimicrobial activity: Bacteroides fragilis, B. melaninigenicus, Prevotella, Fusobacterium spp, anaerobes gram-negatives cocci and gram-positive cocci, Cl. perfringens, Giardia lamblia, Entamoeba histolytica, Trichomonas vaginalis Tinidazole is effective as single dose regimen for treatment of trichomoniasis and giardiasis. Other nitroimidazole compounds are ornidazole, carnidazole and secnidazole. FOSFOMYCINE Fosfomycine is active against Staphylococcus (even the methicillin-resistant Staphylococcus) and Gram negative bacilli. Recommended doses: - 8-12 g/d, iv (adults) - 100-200 mg/kg/d (child) FLUOROQUINOLONES Table 24. Classification and antimicrobial spectra of fluoroquinolones Biologic classification of fluoroquinolones GroupI: Limited spectrum Nalidixic acid Flumequine Oxolinique acid Piromedique acid Pipemidique acid Cinoxacine Group II: Large spectrum Pefloxacin Enoxacin Norfloxacin Ciprofloxacin Fleroxacin Lomefloxacin Ofloxacin Group III: Extended spectrum Temafloxacin Tosufloxacin Moxifloxacin Grepafloxacin Clinafloxacin Gemifloxacin Trovafloxacin Levofloxacin Gatifloxacin Sitafloxacin Microbiologic classification ENTEROBACTERIACEAE Enterobacteriaceae and: H. influenzae Neisseria spp Coagulase negative staphylococcus Intracellular pathogens Mycoplasma spp P. aeruginosa Acinetobacter spp Vibrio holerae M. tuberculosis M. leprae Gpoup II spectra + S. pneumoniae Streptococcus spp +/ANAEROBES Caution for the use of FQ in children KETOLIDES The most prominent member of ketolides used in therapy is TELITHROMYCINE. The antibiotic spectrum includes: Gram – positive cocci , including: Macrolides – resistant -Streptococcus pyogenes, S. aureus, S. pneumoniae Gram – negative pathogens: H. influenzae, Moraxella catarralis Legionella pneumophila Mycoplasma pneumoniae Chlamydia pneumoniae OXAZOLIDINONES Oxazolidinones belongs to a new family of antimicrobial agents. The first compound introduced in therapy is LINEZOLID. The spectrum of activity is mainly represented by GRAM – POSITIVE BACTERIAL PATHOGENS: Methicillin – resistant S. aureus (MRSA) S. epidermidis Penicillin – resistant pneumococci (PRP) Vancomycin – resistant enterococci (VRE) Vanco – intermediate strains (VISA) Indications: Community and hospital – acquired pneumonia Skin infections Other infections with gram positive – resistant germs. Dose: 600 mg. x 2 / day, or 25 mg/kg/day Excretion: Liver 70% + Renal 30%. SULFONAMIDES AND TRIMETHOPRIM Table 25. Antimicrobial spectra of sulfonamides DRUG ANTIMICROBIAL ACTIVITY Sulfadiazine Gram positive organisms (S. aureus, S. Sulfisoxazole pneumoniae, S. pyogenes, Sulfametoxazole Enterococcus fecalis, Corynebacterium Sulfadoxine diphteriae, Listeria monocytogenes, Bacillus antracis) Gram negative organisms (E. coli, Klebsiella, Salmonella, Serratia, Shigella, H. influenzae, N. meningitidis) Other: Chlamydia Nocardia asteroides. trachomatis, TRIMETHOPRIM is a dihydrofolate reductase inhibitor, which is available as a single agent and in combination with sulfamethoxazole (trimethoprim 80 mg; sulfamethoxazole 400 mg). Clinical use: - urinary tract infections - respiratory tract infections (acute bronchitis) - gastrointestinal infections (typhoid fever, diarrhea cased by ETEC) - gonorrhea, brucellosis, periodontal infections, nocardiosis. - Pneumocystis carinii infections ANTIVIRAL DRUGS Table 26. Characteristics of antiviral drugs ANTIVIRAL DRUG ACYCLOVIR MECHANISM VIRUS DOSES OF ACTION AFFECTED Acycl. triphosphate inhibits H. simplex, varicella-zoster, 10 mg/ viral DNA polymerase. cytomegalovirus kg/d,po/iv. !ADVERSE EFFECTS: reversible nephropathy, GI disturbances, phlebitis, headache, encephalopathy. Same as Acyclovir Same as Acyclovir VALACYCLOVIR ! A.E: Same as for Acyclovir + Thrombotic microangiopathy. Ganciclovir triph.inhibits Cytomegalovirus, 5 mg/kg/d GANCICLOVIR viral DNA polymerase. possible:HS, VZ, HHV8 ! A.E: Bone marrow suppression (granulocytopenia) – common, renal insufficiency, fever, headache, phlebitis, rash, encephalopathy Pencicl.triph inhibits PENCICLOVIR viral DNA polymerase. Only available topically, with no appreciable systemic absorbtion! ! A.E: None. Same as Penciclovir. Herpes simplex, varicellaFAMCICLOVIR zoster ! A.E: Headache, nausea, diarrhea, interactions with drugs inhibiting or requiring hepatic oxidation – uncommon. Inhibits viral DNA polymerase, Cytomegalov, acyclovir 60mg/kg/d FOSCARNET reverse transcriptase. resistant HSV, VZV every 8h ! A.E: Renal failure, electrolyte imbalance ( esp. hypocalcemia) , nausea, vomiting – common, anemia, genital ulcers, seizures – uncommon. Interference with viral Lassa fever, hantavirus, 0.5/0.6 g RIBAVIRIN messanger RNA. hepatitis C (chronic) x2 /d, po ! A.E: Anemia – common, skin, eye and upper airway irritation, bronchospasm – common Inhibition of viral DNA and Hepatitis B (chronic cases), 100 mg/d, reverse transcriptase. HIV type 1 po ! A.E.: Lactic acidosis and severe hepatomegaly with steatosis – rare. Blocks a protein ion channel, Influenza A virus 100mg/d, AMANTADINE altering the intracellular pH. 2.2mg/k/d ! A.E.: Nausea and anorexia – common, CNS dysfunction – uncommon, death from overdose – rare. As amantadine As amantadine 200mg/d, RIMANTADINE 5mg/kg/d ! A.E.: Nausea and anorexia, CNS dysfunction – less frequent than with amantadine. Interference with viral Hepatitis B and C (possible INTERFERON ALFA protein synthesis. D), HHV8, Papillomavirus DOSES: B hepatitis: 5 millions units/d or 10 mill U 3 times/week, sc /im for 16-24 wks; C hepatitis: 3 mill.U 3 times weekly, subcutaneously /im, for 24-48wks ! A E.: Influenza-like symptoms, GI disturbances, CNS dysfunction (including depression) bone marrow suppression – common, autoimmune phenomena – uncommon. LAMIVUDINE SYSTEMIC ANTIFUNGAL AGENTS DRUG Polyenes -Amphotericin B Flucytosine Imidazoles -Ketoconazole -Itraconazole -Fluconazole DOSE 1mg/kg (T=2-2,5g) INDICATIONS Deep mycosis: Candida spp, Cryptococcus neoformans, Aspergillus, hystoplasma, blastomyces 150 mg/kg/d (in 4 doses) Cryptococcosis, candidiasis (renal, CNS, eye), chromomycosis 400 mg/d qd(>400mg/d Chronic cutaneous candidiasis, if meningitis) coccidioidomycosis, histoplasmosis, blastomycosis 200 mg/d bid Candidiasis vaginitis 200mgx3/d the first 3 Deep mycosis days 50-100 mg once daily Oropharyngeal candidiasis 100-400 mg Esophagial candidiasis 400 mg/d-2 mo (after the Cryptococcal meningitis initial therapy with Ampho B+flucytosine) T= total dose. STREPTOCOCCAL INFECTIONS Egidia Miftode Morphology and identification Table 1: Characteristics of medically important streptococcus Name Streptococcus pyogenes Streptococcus agalactiae Group specific substance A Hemo -lysis Habitat Beta Throat Skin B Beta Female genital tract Colon Enterococcus faecalis D (and other enterococci) None, alpha Streptococcus bovis D (non enterococcus) None F (A,C,G) Beta Streptococcus anginosus (S.inter- and medius, S.constellatus, untypable S milleri group) Usually not Alpha typed or none untypable Viridans streptococci Usually not Alpha typed or none untypable Streptococcus None Alpha Colon Throat, colon, female genital tract Throat colon, female genital tract Mouth throat, colon, female genital tract Throat Common and important Diseases Pharyngitis, impetigo, rheumatic fever, glomerulonephritis Neonatal sepsis and meningitis Abdominal abscess, urinary tract infection, endocarditis Endocarditis, common blood isolate in colon cancer Pyogenic infections, including brain abscesses Not well defined Dental caries (S.mutans), endocarditis, abscesses (with many other bacterial species) Pneumonia, pneumoniae Peptostreptococcus (many species) None None. Alpha Mouth, colon, female genital tract meningitis, endocarditis Abscesses (with multiple other bacterial species) Classification of streptococci of particular medical interest Streptococcus pyogenes. Streptococcus agalactiae. Groups C and G Enterococcus faecalis (E. faecium, E. durans).. Streptococcus bovis. Streptococcus anginosus. Groups E, F, G, H and K-U streptococci.. Streptococcus pneumoniae.. Viridans streptococci. Include S. mitis, S. mutans, S. salivarius, S. sangius and others. -genesis of dental caries. Peptostreptococcus. in mixed anaerobic infections in the abdomen, pelvis, lung or brain. Functional Classes of Fitness Determinants a) Adherence and colonization Possible virulence and transmissibility determinants of group A streptococci: M and M-like protein (binds to membrane cofactor protein on keratinocytes and fibronectin); Hyaluronic acid capsule (fibronectin and fibrinogen binding, invasion) Streptokinase (converts plasminogen to plasmin) Cysteine protease (cleaves fibronectin and degrades vitronectin) b) Avoidance of host defenses Antiphagocytic properties (M proteins) Anticomplementary properties Cysteine protease function. Cytolysins. Streptolysin O Streptolysin S is a potent cytolytic toxin. Superantigens. Group A streptococci secrete a variety of other biologically active proteins referred to as superantigens because of their profound effects on the immune system In group A streptococci there are six known superantigens: SPE A, B and C; SPE F (mitogenic factor) Streptococcal superantigen Str.pyogenes mitogen. The SPEs share many biological properties, including: fever induction, enhance susceptibility to endotoxic shock. Diseases attribuable to invasion by beta-hemolytic group A streptococci Group A streptococci may be classified clinically as the agents of pharyngitis (throat strains), pyoderma (skin strains), rheumatic fever, glomerulonephritis (pharyngeal or skin strains), and erythema (pharyngeal or skin strains). Erysipelas. Puerperal fever. Sepsis. . Fulminant, invasive group A streptococcal infections, with streptococcal toxic shock syndrome are characterized by shock, bacteremia, respiratory failure, and multiorgan failure. Death occurs in about 30% of patients. Group B streptococci are part of the normal vaginal flora in 5 – 25% of women. Group B streptococcal infection during the first month of life may present as fulminant sepsis, meningitis, or respiratory distress syndrome. Poststreptococcal diseases (rheumatic fever, glomerulonephritis) Following an acute group A streptococcal infection, there is a latent period of 1 – 4 weeks, after which nephritis or rheumatic fever occasionaly develops Acute glomerulonephritis. Rheumatic fever. SCARLET FEVER Egidia Miftode Epidemiology The mortality has declined from 72% in the preantibiotic era to 7 to 27%. Pathogenesis There are three mechanisms involved in the production of scarlet fever: toxic, septic and immunologic. 1. The streptococcal erythrogenic toxin. 2. As a result of soft tissue infections may occurs otitis, sinusitis, adenitis and bacteremia. 3. Immune mechanism: antibodies against different streptococcal antigens ( Clinical manifestations Incubation period is usually 3-6 days. The onset is abrupt with fever, headache, dysphagia, vomiting, with approximately 3 days duration. Exanthem – Characteristic: Filatov’s mask: Pastia’s lines: The erythema abates in 7-9 days. Enanthem consists of: Characteristic appearance of tongue Exudative or erythematous pharyngitis and tonsillitis, and very rarely, ulcerative aspect of tonsillitis. Descuamation period starts after 7-14 days of illness Laboratory features 1. Throat culture - positive for group A streptococci; 2. Rapid antigen detection tests in throat swab; 3. White blood cell count reveals leukocytosis, hypereosinophilia, neutrophilia; 4. Increased ESR 5. Intracutaneous administration of erythrogenic toxin elicits local erythema (positive Dick test – not used at the present time). Differential diagnosis 1. Other infectious causes of tonsillitis: .. 2. Different eruptive disease: … Kawasaki Complications of scarlet fever Suppurative complications: Nonsuppurative complications: glomerulonephritis. acute rheumatic fever, acute Treatment To prevent primary attacks of rheumatic fever, treatment should ensure penicillin levels for at least 10 days. This can be achieved by 7 days of penicillin G (2-4 million IU/day) followed by 3 administration of benzathine penicillin (every 7 days). If penicillin allergy is suspected, the drug of choice is erythromycin (30-40 mg/kg/day). MEASLES Egidia Miftode Measles is a contagious disease characterized by a prodrome of fever, cough, coryza, and conjunctivitis, followed by an erythematous, maculopapular, confluent rash and a pathognomonic enanthem (Koplik spots). Etiology measles virus, member of the genus Morbillivirus (family Paramyxoviridae). Measles virus is a spherical, enveloped RNA virus, Infected cells may also develop intranuclear and intracytoplasmic inclusion bodies (a frequent pathologic feature of persistent CNS infections). Pathogenesis Infection is spread between individuals by the respiratory route. In the second day, primary viremia occurs On the 5th to 7th day after inoculation, secondary viremia occurs Between the 11th and 14th days after inoculation the greatest viral content is noted; this is the moment when prodrome starts followed by the occurrence of exanthema. Clinical manifestations Incubation period is 10-14 days. The prodromal stage (lasts for 3-4 days) - is characterized by: fever, malaise, cough, coryza conjunctivitis photophobia diarrhea. Koplik spots are the pathognomonic lesions of the measles: Sore throat and gingivitis are another two manifestations of enanthem. The rash (persists for 6-7 days) appears 3-4 days after the onset the lesions are light, pink at the beginning (lesions blanched with pressure), then, they become confluent; maculopapular eruption begins behind the ears, involves the upper part of the neck; spreads centrifugally, the exanthem begins to fade after 3 days of evolution the end of the exanthem is marked by a fine in the same period physical examination can reveals pharyngitis, enlargement of cervical lymph nodes and fever Clinical syndromes of measles 1. Atypical measles It’s a form of measles that occurs in children with a previous administration of measles vaccine. Measles virus can not be isolated, but antibody levels are very high. 2. Modified measles Modified measles is a mild form that occurs in partially immune persons: The distinguishing features are: a longer incubation period and a minimal prodromal period. 3. Measles in pregnant women In a pregnant woman, measles can lead to spontaneous abortion and stillbirth. 4. Measles in immunocompromised patients In immunocompromised patients measles infection evolves as a severe, frequently fatal disease.. Complications Complications may be consequences of viral infection or secondary to bacterial infections: 1. Otitis media, mastoiditis, laryngitis, laryngotracheitis; 2. Pulmonary involvement: 3. Neurologic complications: a. Post infectious encephalomyelitis b. Measles inclusion-body encephalitis c. Subacute sclerosing panencephalitis. Laboratory diagnosis Serological exams: ELISA and hemagglutination-inhibition assay. Virus isolation from nasopharyngeal secretions is not a routine technique. Differential diagnosis rubella, scarlet fever erythema infectiosum roseola infantum enteroviral infection Epstein-Barr virus exanthema viral hepatitis drug-induced exanthema trichinosis. Treatment Bed rest must continue at least 8-10 days after the appearance of rash. Infection is contagious 5 days before and another 5 days after the appearance of rash. Treatment consists, primary, of symptomatic relief. If bacterial superinfection occurs antibiotics are indicated. Prevention Passive immunization with human immune globulin, intramuscularly, within 6 days after exposure, is recommended in children under 1 year of age, in chronically ill patients, pregnant women, immunosuppressed patients. Active immunization Two doses of vaccine are recommended for all the children. RUBELLA Egidia Miftode Rubella (German measles or 3-days measles) is an acute febrile illness characterized by a rash and posterior auricular and suboccipital lymphadenopathy that affects children and young adults. Infection during early pregnancy may result in serious abnormalities of the fetus, referred to as the congenital rubella syndrome. Rubella virus, a member of the Togaviridae family, is the sole member of the Rubivirus genus. Acquired rubella Pathogenesis Infection occurs through the mucosa of the upper respiratory tract. Clinical Findings Incubation takes 2-3 weeks. The rash starts on the face, extends over the trunk and extremities and lasts 1-5 days. Ocasionally, an enanthem consisting of small, red macules on the soft palate precedes/accompanies the rash. Posterior auricular and suboccipital adenopathy Rare complications include thrombocytopenic purpura, myocarditis, Guillain-Barre syndrome, bone marrow aplasia and encephalitis. Laboratory diagnosis isolation of virus evidence of seroconversion. Differential diagnosis: scarlet fever, enterovirus infection, measles, adenovirus infection, human parvovirus B19 disease, sunburn, allergic rash, infectious mononucleosis. Treatment Rubella is a mild, self-limited illness and no specific treatment is given. Laboratory-proved rubella in the first 3-4 months of pregnancy is almost uniformly associated with foetal infection; therapeutic abortion is the only means of avoiding the risk of malformed infants in such cases. Prevention Attenuated live rubella vaccines have been available since 1969 as a single antigen or combined with measles and mumps vaccine. Vaccinated children pose no threat to mothers who are susceptible and pregnant. The vaccine induces immunity in at least 95% of recipients and will endure at least 10 years. Congenital Rubella Syndrome Pathogenesis Maternal viremia associated with rubella infection during pregnancy may result in infection of the placenta and fetus. Mother may be either symptomatic or asymptomatic. Clinical Findings 1. Transient effects in infants 2. Permanent manifestations congenital heart diseases total or partial blindness growth retardation; failure to thrive, hepatosplenomegaly, thrombocytopenic purpura, anemia, osteitis and meningoencephalitis. 3. Developmental abnormalities that appear and progress during childhood and adolescence: 4. Laboratory diagnosis: Demonstration of rubella antibodies of the IgM class 5. Differential diagnosis includes other congenital infections: syphilis, toxoplasmosis, CMV and herpes simplex virus infection. Treatment: There is no specific treatment for congenital rubella. Many abnormalities can be corrected by surgery or may respond to medical therapy. Specific lesions are managed clinically without regard to their etiology. Prevention: To eliminate rubella and the congenital rubella syndrome, it is necessary to immunize women of childbearing age, as well as all school-age children. VARICELLA Egidia Miftode Varicella (chickenpox) is a contagious, benign, acute illness characterized by a generalized vesicular rash, which results from primary infection with varicella-zoster virus (VZV). Etiology VZV belongs to the family Herpesviridae. The VZV genome consists of a double-stranded DNA molecule. Pathogenesis VZV is spread by air droplets from nasopharyngeal secretions. Pathology In the skin, VZV produces ballooning degeneration of epithelial cells in the malpighian layer of the epidermis. Clinical manifestations Incubation period: 15 days (10-20 days) Prodrome symptoms (last for 1-2 days): Exanthem is represented by pink macular (at the beginning) that quickly become papular and develop into vesicle 1 to 4 mm in diameter surrounded by a zone of erythema. The lesions become pustules as inflammatory cells migrate into the vesicular fluid. Enanthem: vesicles also involve mucosal surfaces, and rapidly evolve into shallow ulcerations. Varicella occurring during the first trimester of pregnancy has been associated with congenital abnormalities Neonatal varicella may occur when the mother develops the disease within a period of 5 days before to 2 days after delivery. - Varicella in immunocompromised persons is a serious and potentially fatal infection. Complications 1. Bacterial superinfection, 2. Varicella pneumonia – 3. Neurologic complications: a. Reye’s syndrome b. Cerebellar ataxia c. Encephalitis d. Transverse myelitis e. Aseptic meningitis 4. Other rare complications: pancreatitis. hepatitis, arthritis, Lyell syndrome, Laboratory tests 1. Culture of VZV 2. By electron microscopy and histopathology 3. Detection of VZV antigens in a scrapping obtained from the base of a vesicle; 4. Direct fluorescent antigen detection; 5. PCR 6. Serologic techniques (a fourfold rise in antibody titers is considered diagnostic) Diagnosis 1. The history of exposure/no prior history of chickenpox and the clinical appearance of the exanthem (diffuse vesicular rash); 2. The presence of virus, viral antigens, or virus associated cytopathic effect within the lesions; 3. Documentation of VZV - antibody production. Differential diagnosis Differential diagnosis includes vesicular exanthems caused by: coxackievirus, disseminated HSV infection, diffuse impetigo, or rickettsialpox. UPPER RESPIRATORY TRACT INFECTIONS Egidia Miftode The classification of upper respiratory tract infections includes: 1. The common cold 2. Pharyngitis 3. Epiglotitis 4. Acute laryngitis 5. Acute laryngotracheobronchitis 6. Sinusitis 7. Otitis externa, otitis media and mastoiditis 1. The common cold Viruses associated with the common cold are adenoviruses, parainfluenza virus, influenza virus, rhinoviruses and respiratory syncytial virus. 2. Pharyngitis Table 1. Etiology of pharyngitis Viral: - Rhinoviruses - Adenoviruses - Herpes simplex virus (type 1 and 2) - Parainfluenza virus - Influenza virus - Coxsackievirus A Bacterial: - Streptococcus pyogenes (Group A and C beta-hemolytic streptococcus) - N. gonorrhoeae - Corynebacterium diphteriae - Yersinia enterocolitica - Epstein Barr virus - Cytomegalovirus - HIV-1 - Treponema Chlamydia pneumoniae Mycoplasma pneumoniae and hominis Mixed anaerobic bacterial infection (Vincent’s angina) Clinical manifestations Streptococcal pharyngitis Streptococcal pharyngitis - is characterized by: High fever Severe pharyngeal pain Odynophagia Headache, chills Edema and hyperemia of the tonsills/uvula A patchy, grayish-yellow exudate on the tonsils Tender, enlarged cervical lymph nodes. Complications of streptococcal pharyngitis Suppurative complications: Nonsuppurative complications: acute rheumatic fever, acute glomerulonephritis. Anaerobic pharyngitis (Vincent’s angina) The etiology is represented by mixture of anaerobic bacteria (Fusobacterium necrophorum) and spirochetes. Pharyngoconjunctival fever Adenoviruses are involved in etiology and the manifestations include: malaise, myalgia, sore throat, headache, chills, dizziness, conjunctivitis, erythema and inflammatory exudate. Evolution of temperature is usually 5-6 days. Herpetic pharyngitis It is characterized by the presence of: - vesicle and shallow ulcers on the palate - inflammation and inflammatory exudate - cervical adenopahy. Herpangina Herpangina is primarily seen in children. The etiologic agent are coxsackieviruses. Clinical features consist of: - marked sore throat, dysphagia, anorexia - small vesicles (1-2 mm) on the soft palate, uvula, and anterior tonsilar pillars; after the rupture of vesicles, small and white ulcers appear. Infection with Mycoplasma pneumoniae occurs primarily in collectivities of children and young. Differential diagnosis Infectious pharyngitis must be distinguished from noninfectious conditions: 1. Bullous pemphigoid 2. Systemic lupus erythematous 3. Behcet’s disease 4. Kawasaki disease Laboratory diagnosis Throat culture Rapid antigen detection tests in throat swab Specific serologic tests for infectious mononucleosis Serologic tests for My. pneumoniae, herpes simplex, adenoviruses, etc. Treatment of sterptococcal pharyngitis: 1. The current recommended treatment for this infection is penicillin V 2550 mg/kg/day divided into a 4-dose-per-day schedule for 10 days. 2. Benzathine penicillin (penicillin G) 50,000 u/kg intramuscular 3. If a patient is penicillin-allergic: erythromycin 30 mg/kg/day or azithromycin (given once daily for 5 days only) or clarithromycin (twice daily for 10 days) First-generation cephalosporins Second-generation cephalosporins 3. Epiglottitis Acute epiglottitis is defined as a cellulitis of the epiglottis and adjacent structures that may produces complete airway obstruction. The most frequently etiologic pathogen is H. influenza type B, and occasionally pneumococcus, staphylococcus, streptococcus. H. influenzae epiglottitis may be associated in a large proportion of cases with bacteremia and sepsis, with different secondary location of infection. Clinical manifestations Onset is abrupt, marked by fever, irritability, dysphonia, dysphagia, followed by respiratory distressLaryngoscopy reveals a “cherry-red” epiglottis. Laboratory features Leukocytosis with neutrophilia Positive cultures of blood and epiglottis Radiograph of the lateral neck shows enlarged Differential diagnosis includes: croup, dyphteria, angioneurotic edema, foreign body aspiration, etc.. Therapy: - intravenous therapy with antibiotics such as: cefotaxime (100180mg/kg/day), ceftriaxone (80-100mg/kg/day) or amocicillin-clavulanic acid(200mg of amoxicillin/kg/day) for 7-10 days. 4. Acute laryngitis Etiology: - viruses – - bacteria – - fungi –. Clinical manifestations: recent onset of hoarsness or episodes of aphonia. Examination of larynx reveals hyperemia of vocal folds. Differential diagnosis: croup, acute epiglottitis, supraglottitis, bacterial tracheitis, voice abuse, gastroesophageal reflux disease, laryngeal malignancy. Antibiotics are not routinely recommended. 5. Acute laryngotracheobronchitis (croup) Acute laryngotracheobronchitis is a viral infection that consists of inflammation in the subglottic area. Etiology: parainfluenza viruses, influenza A, B viruses, respiratory syncitial virus, adenovirus, rhinovirus, enterovirus, and rarely, Mycoplasma pneumoniae. Clinical manifestations The croup is preceded by an upper respiratory tract infection. . Laboratory findings: Differential diagnosis - Non infectious causes of stridor: - Bacterial epiglottitis Complications: Therapy: humidification devices of the airway, good supportive care, corticosteroids. 6. Sinusitis The most common bacteria are: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Streptococcus pyogenes, and Staphylococcus aureus. Anaerobic bacteria ,Fungal sinusitis , Viruses may also cause sinusitis. Diagnostic modalities include fiberoptic nasal endoscopy, CT scans, and plain x-rays. Amoxicillin-clavulanic acid, trimethoprim/sulfametoxazole, amocillin Azithromycin/Clarithromycin (if penicillin allergy); Levofloxacin/moxifloxacin (when Penicillin-Resistant Pneumococcus is suspected); 7. Otitis Media Etiological agents the most frequently seen are: S. pneumoniae, H. influenzae, M. catarrhalis, viruses (respiratory syncitial virus, rhinoviruses) Diagnosis is suggested by: Hearing loss Ear pain Fever Delayed speech development in children Therapy Amoxillin or amoxicillin-clavulanic acid Cefuroxime, ceftriaxone (50 mg/kg/day) Clindamycin (if failure of treatment after 3 days). INFLUENZA V. Luca, Egidia Miftode The Orthomyxoviridae (influenza viruses are known: Type A; Type B – Type C Three immunologic types Etiology The HA protein. The antigenicity of NA, The standard nomenclature system for influenza virus isolates includes the following information: type, host of origin, geographic origin, strain number, year of isolation (example: A/Hong Kong/03/68(H3N2). So far, 14 subtypes of HA (H1-H14) and nine subtypes of NA (N1-N9) in many different combinations have been recovered. Antigenic drift and antigenic shift . Minor antigenic changes are termed antigenic drift; Major antigenic changes in HA or NA, called antigenic shift, Influenza virus replication Epidemiology The three types of influenza vary in their epidemiologic patterns. Influenza C is least significant: it causes mild, sporadic respiratory disease, but not epidemic. Influenza B sometimes causes epidemics, Influenza type A can causes around the world massive epidemics called pandemics. Pathogenesis Influenza virus spreads from person to person by airborne droplets or by contact with contaminated hands or surfaces. Clinical findings Uncomplicated Influenza Incubation period: 1-4 days. Symptoms usually appear abruptly and include: chills, headache, dry cough- respiratory symptoms typically last another 3-4 days. The cough and weakness may persist for 1-3 weeks. high fever- lasts 3 days generalised muscle aches, malaise and anorexia Complications Pneumonia Reye’s Syndrome :. Early signs: persistent/continuous vomiting loss of energy irritability fluctuating personality changes confusion As the encephalopathy becomes more severe, extreme irritability, agitation, delirium, convulsions, and coma may develop. Laboratory findings hyperammoniemia elevated levels of alanin aminotransferase and aspartat aminotransferase prolonged prothrombin time hypoglycemia hyperlactatemia acid-base disorders CSF – with <8cells/mmc, and normal level of protein and glucose Differential diagnosis Treatment - Glucose administration - Antiedematous drugs, diuretics - Fresh frozen plasma/fresh blood (if bleeding occurs) - Corticosteroids - The mortality rate is high (10-50%). Other complications: sinusites, myocarditis, pericarditis, cardiac failure, renal failure, neurological complications. Immunity Immunity to influenza is long-lived and subtype-specific. Laboratory Diagnosis Diagnosis of influenza relies on: 1. isolation of the virus; 2. identification of viral antigen or viral nucleic acid in the patient’s cells, or 3. demonstration of a specific immunologic response. Other tests are: ELISA and RIA. Paired acute and convalescent sera are necessary, because normal individuals usually have influenza antibodies. A fourfold or greater increased in titer must occur to indicate influenza infection. Treatment Amantadine and rimantadine, Zanamivir and Oseltamivir A. All people at risk in whom influenza develops B. Persons with severe influenza C. For persons who wish to shorten the duration of illness. Prevention Inactivated viral vaccines The vaccine is usually a cocktail containing two influenza A subtypes (H1N1, H3N2) and a type B virus of the strains isolated in the previous winter’s outbreaks. Annual influenza vaccination is recommended for high-risk groups.: Persons >50 years old those with either chronic heart or lung disease, adult and children with asthma, or metabolic or renal disorders, immunossuppression, hemoglobinopathy residents of nursing homes; persons who might transmit influenza to high-risk groups : - medical personnel, - employees in chronic care facilities, - household members. MUMPS Dr. Egidia Miftode Mumps is an acute viral disease characterized by nonsuppurative swelling and tenderness of the salivary glands. Etiology Mumps virus belongs to paramyxoviridae family. Epidemiology The virus is spread by infectious saliva or by urine. Neonates are protected by transplacental maternal antibodies. Clinical manifestations Incubation period: 14-25 days. Prodromal symptoms (3 days): fever, headache, malaise. Glandular involvement: - The onset of parotitis: Submandibular/sublingual glands involvement (10% of cases) - Epididymo-orchitis Oophoritis – Neurologic manifestations 1. CSF pleoocytosis - Meningitis 2. Encephalitis 3. Other features in mumps are: Renal function abnormalities (>60%); ECG abnormalities (5-15%); Pancreatitis – Thyroid inflammation: Complications 1. Myocarditis – is very rare 2. Arthritis. 3. 4. 5. Hemolytic anemia, trombocytopenia; Deafness with uni, or bilateral involvement; In pregnant women (with gestational viral infection): fetal death is common during the first trimester. low birth weight endocardial fibroelastosis juvenile diabetes mellitus. Laboratory features Diagnosis is based on: history of exposure, parotid swelling and tenderness, constitutional symptoms. Differential diagnosis Parotid swelling must be differentiated by: Other infectious causes: Noninfectious causes: Extraparotid causes: Prognosis is generally good, except severe forms of encephalitis, myocarditis, glotic edema. Lethality is approximately 0,01%. Treatment Treatment is entirely symptomatic: analgesics for orchitis or pancreatitis, drugs against vomiting, etc. Patients should avoid acid food, the diet must be light, with a good hydration. The vaccine contains a live mumps, virus and may be administrated alone or in combination with measles and rubella vaccines. INFECTIOUS MONONUCLEOSIS Egidia Miftode Infectious mononucleosis is an acute illness characterized by fever, pharyngitis, lymphadenopathy, and mononuclear leukocytosis with atypical lymphocytes. Etiology The Epstein-Barr virus (EBV) Pathogenesis EBV infects B lymphocytes and epithelial cells in the oropharynx and cervix. During primary infection, EBV-infected B-cells undergo lytic infection with production of virus or express the full complement of latent viral proteins. The latter cells are kept in check by natural killer and cytotoxic T cells, which may appear as “atypical lymphocytes” on the peripheral blood smear. Some latently infected cells undergo lytic replication in the oropharynx, resulting in production of virus with shedding the virus into the saliva. Clinical manifestations Incubation period: 30-50 days. Characteristic triad consists of: fever (75% of cases), pharyngitis (84%), and lymphadenopathy (94%). Other common signs and symptoms are: splenomegaly (50%), hepatomegaly (10%), palatal petechiae (10%), rash (10%), jaundice (10%) associated with sore throat, headache, anorexia, abdominal pain, nausea, chills, myalgia. A morbilliform rash As a result of congenital infection an embriopathy may occasionally result: Complications Neurologic complications. 1. Haematologic complication: 2. Hepatitis, myocarditis, splenic rupture, genital ulcers. Laboratory tests a. Hemoleucogram shows a mononucleosis syndrome: Leukocytosis an absolute increase in the number of peripheral mononuclear cells atypical lymphocytes (>10%) which are primary T cells responding to the EBV-infected cells. b. Elevated serum aminotransferase levels c. Serological tests. The humoral immune response to EBV infection involves both viral-specific and nonspecific antibodies. Nonspecific antibodies: Paul-Bunnell-Davidson test – Three specific antibodies to EBV antigens are diagnostically important, and the antigens are: VCA – viral capsid antigen EA – early antigen EBNA – EBV nuclear antigen(table 1) Table 1. Diagnostic tests in infectious mononucleosis Antibody Heterophil Onset Before or at time of symptoms Duration Weeks to months VCA-IgM VCA-IgG EBNA EA Before or at time of symptoms Before or at time of symptoms 3-4 weeks after onset peaks 3-4 weeks after onset 4-8 weeks Lifelong Lifelong Months to years d. Isolation of the pathogen -EBV culture is not a routine method -demonstration of EBV genoma by PCR and of EBV antigen by immunoblot techniques. Other clinical syndromes produced by EBV infection Chronic active EBV infection - is a rare disorder X-Linked Lymphoproliferative Disease -. Cancers associated with EBV 1.Nasopharyngeal carcinoma – 3.Hodgkin’s disease. 4.Lymphoproliferative disease – 5.Other tumors: Treatment No specific therapy is indicated for most patients with infectious mononucleosis. Corticosteroid therapy is recommended for patients with severe complications: DIPHTHERIA Egidia Miftode Diphtheria is an acute disease manifested by both local infection of the upper respiratory tract and the systemic effects of a toxin, which are most notable in the heart and peripheral nerves. Etiology The etiologic agent is the principal human pathogen of the Corynebacterium group, C. diphtheriae, an aerobic gram-positive bacillus with irregular shape. Pathology All human tissues may suffer by the toxin because all human cells have receptor sites. The diphtheria bacilli within the membrane continue to produce toxin actively. This is absorbed and leads to distant toxic damage, particularly parenchymatous degeneration, fatty infiltration and necrosis in heart muscle, liver, kidneys (tubular necrosis), adrenals, sometimes accompanied by important hemorrhage. The toxin also produces nerve damage (neuronal demyelination), resulting often in paralysis of the soft palate, eye muscles, or extremities. There are 2 phases of diphtheria: the initial local presentation as a severe pharyngitis with tough membranes that can cause suffocation and a late systemic phase caused by the effects of the circulating exotoxin on tissues of the host. Nondiptheria corynebacteria produce localized or systemic diseases Clinical Findings Incubation period is usually less than 1 week. Pharyngitis. Laryngeal diphtheria. Nasal diphteria Cutaneous infection Other organ involvement includes: ears, conjunctiva, cornea. Complications 1. Cardiovascular complications Myocarditis Late myocarditis – 2. Neurologic complications a. Palatal paralysis – b. Oculomotor paralysis – c. Peripheral polyneuritis Laboratory Tests 1. Isolation of C. diphteriae 2. Stained smears show beaded rods in typical arrangement. Diagnosis Differential diagnosis Other pharyngeal diseases: Retropharyngeal and peritonsillar abscesses. A foreign body in the larynx, viral laryngitis Treatment Note: specific treatment must never be delayed for laboratory reports if the clinical picture is strongly suggestive of diphtheria. 1. The imperative in diphtheria treatment is to administer the antitoxin as soon as possible, as it is the only mean to neutralize toxin that has not already bound to cells. The mainstay of therapy is prompt administration of equine diphtheria antitoxin: 20000-100000 IU, i.v. the test for hypersensitivity consists of administration of one drop of antitoxin diluted 1:10 in one eye. If the antitoxin is administrated in the first day of illness, the mortality is less than 1%. Antibiotics PENICILLIN or ERYTHROMYCIN 2. Supportive care and maintenance of an airway 3. Strict bed rest during the acute phase of diphteria. Prevention Diphtheria was the first bacterial disease for which toxic cause was demonstrated and the first to be treated successfully with an antitoxin. In 1913 a vaccine was created, composed of treated diphtheria toxin, called anatoxin, later transformed in diphtheria toxoid. In 1940 a combined vaccine appeared: DTP = Diphtheria toxoid + Tetanus toxoid + Pertussis vaccine. Active immunization in childhood with diphtheria toxoid In the most developing countries, immunization with diphteria and tetanus toxoids and pertussis vaccine was introduced by the late 1970s; in countries with low immunization coverage, diphteria continues to be endemic. WOOPING COUGH Egidia Miftode, Carmen. Corcaci Whooping cough is presently one of the ten most common causes of death from infectious diseases worldwide. Despite a high vaccine uptake, resurgences of this disease have been observed in several countries. Etiology The genus Bordetella consists of the following species: B. pertussis-infects only humans B. parapertussis-cause a similar but milder disease in humans (also isolated from sheeps) B. bronchiseptica-infect pigs rabbits, dogs, and rarely humans. B. avium-is similar with B. bronchiseptica and cause turkey coryza and rare human infection B. hinzii, B. holmesii and B. trematum rarely cause human infections. The human pathogens are small aerobic, nonmotile gram-negative coccobacilly. All species are piliated, aerobic and oxidize aminoacids but do not ferment carbohydrates. The organisms require nicotinamide for growth at an optimal temperature of 35-37°C. B. parapertussis grows slightly faster,produces larger colonies and in peptone agar or in liquid medium produces a characteristic brownish pigment. Virulence factors 1. Agglutinogens. 2. Fimbriae (three major-2, 3, X, and one minor-Y) – 3. Pertussis toxin (PT) 4. Pertactin 5. Filamentous haemagglutinin (FHA) 6. Tracheal cytotoxin (TCT). 7. Dermonecrotic toxin Lipopolysaccharide - produces endotoxin-like effects, sensitisation to histamine, and is pyrogenic. 8. Tracheal colonisation factor 9. Serum resistance factor. B. pertussis. Phisiopathology Roles for these multiple factors in the pathophysiology of pertussis can be considered in the context of a generic sequence of events for an infectious disease: Epidemiology The organism is transmitted by respiratory droplets, with attack rates of 50100%. Clinical manifestations The incubation period is 5-7 days. The catarrhal phase (lasting 7 days) is characterized by nonspecific symptoms: rhinorrhea, mild conjunctival congestion, lacrimation and normal temperature. Paroxysmal phase (last at least 2 weeks): a dry, nonproductive cough with vomiting develops. Rarely, signs of encephalopaty may be seen. The convalescent phase begins with a decrease in intensity of the cough and the frequency of paroxysms. The disease may be atypical in the adult age group and in partially immunized children and adults. Complications Complications of pertussis include: 1. Secondary infections. 2. Physical sequelae of paroxysmal cough. 3.CNS complications. The pertussis encephalopathy may occur in 0,9%, and convulsions in 3%. . Laboratory features The total white blood cell count exceeds 50000 cells/mmc with lymphocytosis. hypoglicemia. Pulmonary consolidation is seen on the chest radiograph of more than 20% of hospital patients. Isolation of organism by culture Another method is direct fluorescein-labeled antibody to detect B. pertussis in nasopharingeal smears. Serological tests DNA hybridization enzyme assay or antibodies Diagnosis The WHO definition of pertussis is based on both a minimum of 21 days of paroxysmal cough and confirmation of infection by laboratory tests. Treatment erythromycin,(and also azithromycin, clarithromycin) – is the drug of choice; aful 2 weeks course should be performed to prevent the relapse. tetracycline trimethoprim-sulfamethoxazole. Chloramphenicol. Corticosteroids should be limited in infants with life-threatening disease. Prevention The vaccine currently recommended by the World Health Organization Acellular vaccine: various pertussis virulence factors have been shown to be more or less protective when used in acellular vaccine (pertussis toxoid, filamentous haemagglutinin, pertactin and fimbriae). ACUTE BACTERIAL MENINGITIS Egidia Miftode Acute meningitis is a medical emergency that requires the utmost in diagnosis and therapeutic skills. The death rate, about 30% have changed little in the last 25 years. Pathogenesis Bacteria reach the meninges through one of the following pathways: 1. hematogenous dissemination from a distant site (nasopharynx, skin, lung, etc) 2. spread from an adiacent suppurative focus of infection (otitis, sinusitis, mastoiditis) 3. a congenital or an acquired structural defect The pathogenic sequence of bacterial neurotropism is as follows: colonization or mucosal invasion intravascular survival crossing of blood-brain-barrier survival within CSF:. Production of the disease in the meninges and brain. Table 1: Factors involved in the pathogenesis and pathophysiology of bacterial CNS infections FACTORS INVOLVED IN THE PATHOGENESIS AND PATHOPHYSIOLOGY OF BACTERIAL CNS INFECTIONS Bacterial factors Fimbriae Capsule Outer-membrane proteins Lipopolysaccharide Cell wall Peptidoglycan Slime Host factors Blocking IgA antibody Decreased CSF immunoglobulin concentrations Decreased CSF complement concentrations Impaired phagocytosis Production of inflammatory cytokines (interleukin –1 , tumor necrosis factor, interleukin-6, interleukin-8, interleukin-10) Production of arachidonic acid metabolites (e.g., prostaglandin E2) Production of platelet-activating factor Production of reactive oxygen species (nitric oxide, superoxide, peroxynitrite) Production of excitatory amino-acids (glutamate, aspartate, glycine, taurine, alanine) Table 2: Pathophysiologic consequences of subarachnoid space inflammation Pathophysiologic consequences of subarachnoid space inflammation Increased blood brain barrier permeability Cerebral edema (vasogenic; interstitial, cytotoxic) Cerebral vasculitis Increased CSF outflow resistance Increased intracranial pressure Altered cerebral blood flow; loss of autoregulation of cerebral blood flow; Cerebral cortical hypoxia; CSF acidosis secondary to glucose utilization by anaerobic glycolysis Increased CSF lactate Decreased CSF glucose Neuronal injuries Cranial and spinal nerve dysfunction Encephalopathy Pathology On gross examination there is purulent exudate which is most abundant in the cisterns, at the base of the brain, and over the convexities of the hemispheres. Etiology Table 3 Etiology related to history/physical examination Historical data/ physical examination Hospital acquired Contact with rodents/domestic animals Upper respiratory infections Brain abscess Trauma -closed skull fracture -open skull fracture -CSF othorrhea and rhinorrhea Neurosurgery Underlying conditions Complement deficits Microorganisms GNB, Staphylococci Leptospires Pneumococci, meningococci Anaerobes -pneumococcus, GNB -GNB, staphylococci -pneumococcus, GNB, staphylococcus Staphylococci, GNB Pneumococcus Meningococci Clinical manifestations Initial symptoms could be: fever, headache, lethargy or altered level of consciousness, irritability, vomiting, photophobia, respiratory symptoms. The altered mental state may range from simple irritability to confusion, obtundation, or coma. In elderly, meningitis should be suspected if presence of: fever, disorientation, headache, stupor, or coma. Physical examination A. Signs of meningeal irritation: Nuchal rigidity neck stiffnes Brudzinski’s signs: - the nape –of –the – neck sign: - the reciprocal controlateral reflex sign - Kernig’s signs: -first sign-; second signB. Presence of skin eruptions: petechiae, purpura; C. Cardiovascular manifestations: bradycardia, hypertension or low blood pressure D. Respiratory features: respiratory failure of central cause E. Focal neurologic signs: cranial nerve palsies with abnormalities of ocular motility hemiparesis visual fiel defects ataxia. In neonates and infants the signs and symptoms of meningitis may be very subtle: fever, irritability, lassitude, confusion, seizures, diarrhea, full fontanella, Lesage sign Complications 1. Subdural effusions (empyema)2. Obstructive or communicating hydrocephalus: Brain abscess, cerebritis, cortical infarction, ventricular empyema, superior sagital sinus thrombosis: 3. Cranial nerve dysfunction:. 4. Neurosensorial abnormalities: 5. Hemiparesis, quadriparesis Sequelae Neonates and infants more frequently have long-term sequelae: Permanent sensorineural hearing loss Blindness Hemiparesis Quadriparesis Mental retardation Late-onset epilepsy Diabetes insipidus Seizure disorders Diagnosis of meningitis White blood cell count The normal WBC count in the CSF is 0-10 per cubic mm (does not contain PMN). In bacterial meningitis there are > 100 WBC/ mm3 (frequently >1000WBC/mm3) (table 3). Differential count shows a predominance of PMN Table 4. Typical CSF findings in bacterial versus aseptic meningitis CSF parameter WBC Glucose Protein Lactate Gram’s stain Opening pressure Bacterial meningitis >10 to <10000/mm3 PMN predominate <40 mg/dl >50 mg/dl Aseptic meningitis 50-2000/mm3 Lymphocytes predominate >45 mg/dl Normal or slightly elevated Normal Negative >3,8mmol/l + in 70-90% of untreated cases Normal or slightly >180mmH2O elevated . Microbiological data for the etiological diagnosis of bacterial meningitis Different approaches for the diagnosis of bacterial meningitis: 1. Classical bacteriologic diagnosis: Gram stain Cultures 2. Detection of bacterial components metabolites: a).physico-chemical -gas –cromatographie -mass-spectrometrie b).biochemical: -detection of an endotoxin by limulus test -detection of the genome(hybridation , polymerase chain reaction) c).immunological- detection of antigens in CSF, serum, urine by: -counter-imunoelectrophorese -latex agglutination/coagglutination -ELISA, RIA d).Detection of an immune-response 3. Non specific biochemical reactions . Direct examination The cultures The cultures-are performed at 37 C with a daily follow up of cultures at lest Determination of bacterial genoma: Immunological tests Differential diagnosis 1. 2. 3. 4. Meningeal reaction Brain abscess, subdural empyema) Subarachnoid hemorhage Noninfectious conditions: Systemic lupus erythematosis, sarcoidosis Non-steroidal anti-inflammatory drugs, cotrimoxazole Spinal trauma/spinal tap, myelography Increased level of blood ureea Neoplasms: glioma, leukemia, craniopharyngioma 5. Bacterial endocarditis 6. Spirochetoses 7. Viral meningitis 8. Fungal meningitis 9. Protozoan and parasitic meningitis 10.Brucellosis Treatment Table 4: Antibiotics recommended for empirical therapy in patients with suspected bacterial meningitis who have a nondiagnostic Gram's stain of cerebrospinal fluid Group of patients Immunocompetent With impaired cellular immunity With head trauma, neurosurgery, or cerebrospinal fluid shunt Likely Pathogen Choice of antibiotic S.agalctiae, E.coli, L.monocytogenes N.meningit., S.pneum., H.influenzae S.pneum., N.meningit. S.pneum.,L.monocyt ., gram neg.bacilli L.monocyt./gramneg bacilli Staphylococci, gramneg.bacilli, S.pneumoniae Ampicillin (2) + broadspectrum cephalosporin Broad-spectrum cephalosporin (3) Broad-spectrum cephalosporin (4) Ampicillin (5) + broadspectrum cephalosporin (4) Ampicillin + Ceftazidime Vancomycin Ceftazidime + Treatment of increased intracranial pressure: - head of the bed elevated - mannitol - phenobarbital Glucocorticoids: dexametasone is indicated in children more than two months of age who have bacterial meningitis, in Haemophilus influenzae meningitis, Meningococcal meningitis The meningococcus is a gram-negative diplococcus which lives only in the human orofarynx Fulminating meningococcemia (Waterhouse-Friederichsen syndrome) This syndrome is characterized by the following: Sudden onset of a febrile illness Extensive petechial hemorrhages in the skin and mucous membranes Cardiovascular collapse Disseminated intravascular coagulation Table 5. Prognosis score in fulminating meningococcemia Clinical/laboratory features WBC count in CSF <20/mm3 Cardiovascular collapse Petechial hemorrhages appeared within the first 12 hours of the disease Absence of blood leukocytosis ESR=normal High rectal temperature Thrombocytopenia Score 1 1 1 1 1 1 1 1 Haemophilus influenzae meningitis Haemophilus species are small, gram-negative pleomorphic coccobacilli. Almost all invasive infections are caused by H. influenzae serotype b (Hib). Hib is the most common cause of bacterial meningitis between the neonatal period and age 6 years. In older patients Hib meningitis occur when predisposing factors exist: Otitis media Other parameningeal focci of infection CSF leak Head trauma An immunocompromised disease. Hib meningitis are seen more frequently in winter, following an influenzalike syndrome. Onset could be: acute (like in other forms of meningitis) with coma seizures respiratory abnormalities purpuric/petechial skin eruptions Children with Hib meningitis usually present with an illness that have been progressive over 24-72 hours; some children may have a fulminant illness that develops over several hours. A common complication of Hib meningitis is subdural empyema. Treatment First-intention therapy: third generation cephalosporins (cefotaxime, ceftriaxone) or ampicillin with chloramphenicol. If H. influenzae is not a beta-lactamases producer strain: Ampicillin 200-400mg/kgc/day. Duration of therapy is 2-3 weeks (a condition being CSF protein<0,4g/l). Pneumococcal meningitis Streptococcus pneumoniae, a gram-positive diplococcus is the major cause of meningitis in adults over 15 years of age, accounting for 30-50% of cases. Predisposing factors are: Pneumonia, sinusitis, endocarditis Old or recent head trauma, CSF leak Splenectomy Sickle hemoglobinopathy Bone marrow transplant alcoholism Treatment Penicillin G: 300 000-400 000U/kgc/day, for children and 15 mil U/day in adults Ampicillin: 400 mg/kgc/day for children and 8-12 g/day in adults For S. pneumoniae with low sensitivity to PG: third generation cephalosporins (cefotaxime-100mg/kgc/day in children and 3-5g/day in adults, ceftriaxone) For Penicillin –resistant-Pneumococcus: Vancomycin: 30-40 mg/kgc/day Rifampin: 10-20mg/day Chloramphenicol (usually, a PRP is also chloramphenicol-resistant): 75100mg/kgc/day in children and 3g/day in adults Duration of treatment is 14 days, but depend also on clinical (absence of fever and other clinical manifestations) and CSF evolution (pleocytosis<30/mm3, and 0% PMN). Staphylococcus aureus Staphylococcus aureus- commonly the result of spread from a site outside the CNS: various abscesses (abdominal, cerebral, epidural, oral), cellulitis, sinusitis, osteomyelitis, decubitus ulcer, infected intravascular grafts. Since the decades of antimicrobial therapy, staphylococcal meningitis has been seen most frequently in patients with underlying CNS pathology: trauma, craniotomy, CSF shunts. The issues of treatment and outcome make for an interesting analysis. Patients with Staphylococcal aureus meningitis should be treated with: high doses of Cefotaxime/Ceftazidime with/without a fluoroquinolone or Rifampin. Vancomycin is reserved for patients who are either allergic to penicillin or have methicillin-resistant organisms as the cause of the meningitis. In this situation must be done a close monitoring of CSF concentrations during therapy. Cefotaxime or Vancomycin could be associated with rifampin in the treatment of S.aureus meningitis, as it may synergistically enhance the activity of the first two agents in vitro. Epidural abscess An epidural abscess represents localized infection between the outermost layer of the meninges, the dura mater, and the overlying skull or vertebral column. Sources of infection are numerous and include: bacterial endocarditis, infected indwelling catheter, urinary tract infection, peritoneal and retroperitoneal infection direct extension of infection from vertebral osteomyelitis occurs in adults and rarely in children. Clinical manifestations A four phase sequential evolution has been described with: (1) localized spinal pain, (2) radicular pain and paresthesias, (3) muscular weakness, sensory loss, and sphincter dysfunction, and (4) paralysis. Positive diagnosis Headache is a common additional complaint. Nuchal rigidity and focal tenderness to percussion are almost universal. The white blood cell count and the erythrocyte sedimentation rate are elevated. CSF evaluation. X-ray films of the spine may show osteomyelitis. Computerized tomography may be helpful, Myelography is the diagnostic procedure of choice. Blood and abscess aspirate should be submitted for aerobic and anaerobic culture. Meningitis due to Listeria monocytogenes This organism is an important cause of neonatal and elderly meningitis. For the neonates the source is the genital tract or subclinical infection of the mother. Early neonatal disease usually presents as sepsis rather than meningitis. L. monocytogenes may causes meningitis in normal adults, which are: diabetic, alcoholic, or immunosuppressed. Clinical presentation includes: fever and headache , increased tendency for focal neurologic deficits and seizures during the initial presentation. clinical picture suggestive of an acute brain-stem disorder or rhombencephalitis, with signs of ataxia, cranial nerve deficits, and nystagmus CSF are characterized by wide ranges for WBC count Tratament Ampicillin is the drug of choice (often combined with gentamicin. For treatment of L.monocytogenes meningitis in patients allergic to ampicillin, intravenous trimethoprim-sulfamethoxazole (TMP-SMZ) is recommended. Meropenem Meningitis with Cryptococcus neoformans Most frequently this type of meningitis occur in AIDS patients (incidence of 2-11%). On pathologic examination is seen a basilar, chronic meningitis that is typically neither thick nor exudative. Classic features are: headache, photophobia, mental status changing and neck stiffness, although they may be absent in many patients with AIDS. Treatment Amphotericin B (0,6-1 mg/kg/day) iv, at least 6 weeks, associated or not with 5-flucytosine (100-150mg/kg\day)-2 weeks followed by oral fluconazole (400mg/day)-8-10 weeks. If, at the end of this period , the CSF culture is negative, the fluconazole doze may be reduced to 200mg/day and continued as secondary prophylaxis. Recurrent bacterial meningitis Table 5: Conditions predisposing to recurrent bacterial meningitis Defects of the stapes footplate, oval window, cohlear aqueduct Giant apical air cell syndrome Basietmoidal or cribriform plate defect Cranial or spinal dermal sinus Meningocele Encephalocele Neurenteric cyst Traumatic or surgical CSF fistulae skull fracture involving paranasal sinuses, cribriform plate or petrous bone postoperative (particularly, following nasal surgery) Immunodeficiency immunoglobulin/complement component deficiency hemoglobinopathy congenital or acquired asplenia leukemia lymphoma Congenital CSF fistulae Meningitis produced by Gram Negative bacilli Approximately 80% of cases of Gram-negative bacillary meningitis occur in conjunction with head trauma or neurosurgical procedures. Meningitis with organism such as E.coli, Klebsiella and Pseudomonas occur later in the hospital course after the use of antibiotics for treatment of other infection or an initial episode of meningitis. It could appear that the overall use of prophylactic antimicrobial agents does not change the incidence of most postoperative bacterial meningitis and may in fact result in the selection of highly resistant gram-negative organisms. Approximately 20% of shunt infections are due to a variety of gramnegative organisms such as: E.coli, Klebsiella pneumoniae and Pseudomonas. Another GNB that is involved more and more in nosocomial meningitis in the presence of instrumentation is Acinetobacter. Many of bacteriaemia due to Acinetobacter are polimicrobial. Meningitis caused by P.aeruginosa represents a special situation because reported mortality rates are approximately 84%. Treatment Third generation cephalosporins (ceftiaxone, cefotaxime, ceftazidime) associated with an aminoglycoside or a fluoroquinolone Piperacillin/Azlocillin with fluoroquinolones Meropenem alone or in association Aztreonam. HYDROCEPHALUS SHUNT INFECTION Hydrocephalus, a condition caused by accumulation of cerebrospinal fluid (CSF) within the cerebral ventricular system, is commonly seen in neurosurgical practice. The usual cause of the accumulation is obstruction to the flow of CSF or failure to absorb it. The incidence of shunt infections varies considerably between centers, from over 30% to less than 1% of operations. Etiology Clinical manifestations In VA shunts, the organism from the lumen of the colonized Treatment The three main goal of treatment of any CNS prosthetic device infection are: 1. minimizing the mortality and morbidity of the infection and it’s treatment 2. maintaining a functioning device if it is still needed 3. resolving the infection; the drug of choice is vancomycin associated with rifampin. The third generation cephalosporins have good anti-gram-negative coverage. Table 5: Recommendations for antibiotic therapy in patients with bacterial meningitis who have a positive Gram's stain or culture of cerebrospinal fluid TYPE OF BACTERIA On Gram's staining Cocci - negative CHOICE OF ANTIBIOTIC Ampicillin Vancomycin + broad-spectrum cephalosporin Broad-spectrum cephalosporin +/aminoglycoside Bacilli -positive -negative Ampicillin/Penicillin G + aminoglycoside Broad-spectrum cephalosporin aminoglycoside + On culture S.pneumoniae Ampicillin/Penicillin Vancomycine + broad-spectrum cephalosporin H.influenzae Ceftriaxone/ Ampicilin+Chloramphenicol N.meningitidis Penicillin G L.monocytogenes Ampicillin + Gentamicin S. agalactiae Penicillin G Enterobacteriaceae Broad-spectrum cephalosporin + aminoglycoside /Fluoroquinolones Meropenem Pseudomonas aeruginosa, Ceftazidime + Acinetobacter aminoglycoside/Fluoroquinolones Meropenem Neonatal bacterial meningitis Many factors are considered to be risk factors for newborn meningitis: Low birth weight (<2500g) Resuscitation at birth Premature rupture of the membrane Mother infection The most frequently agents are: Streptococci group A, B, D (45%), Gramnegative bacilli (Klebsiella, Enterobacter, Echerichia, Pseudomonas) (44%), Listeria monocytogenes (5%), Staphylococcus epidermidis and aureus. Clinical manifestations Early onset disease (onset within 48 hours after delivery) -infants present with symptoms and signs of sepsis: fever, gastrointestinal manifestations (abdominal distension, vomiting) respiratory distress, and lethargy; -CNS specific symptoms are often not detected Late-onset disease (onset later than 7 days after birth and up to 6 weeks of age): fever and presence of CNS dysfunction (seizures). Treatment Ampicillin and cefotaxime/ceftriaxone Ampicillin and an aminoglycoside VIRAL MENINGITIS Egidia Miftode Acute meningeal inflammation produced by viruses is usually characterized by moderate signs and symptoms, low level of WBC in CSF, with lymphocytic predominance, and a benign evolution. Etiology Common causes: Enteroviruses Arboviruses Herpes simplex virus type 2 Uncommon causes: Mumps Human herpes virus type 6 Lymphocytic choriomeningitis virus Human immunodeficiency virus Rare causes: Herpes simplex virus type 1 Varicella-zoster virus Cytomegalovirus Epstein-Barr virus Influenza viruses A and B Measles virus Rotavirus Encephalomyocarditis virus Parvovirus B19. Clinical manifestations ENTEROVIRUSES Pathogenesis The virus traverses the intestinal lining cells, A minor viremia, seeding numerous organ systems: CNS, liver, lungs and heart. The characteristic rash caused by an enterovirus consist of erythematous macules and papules on the face, neck, trunk, and to a lesser degree the extremities. Neonates are at risk for severe systemic illness: For other cathegories of age the symptoms and signs are: -the fever pattern may be biphasic -photophobia, headache, vomiting -rash, diarrhea, myalgias, cough. Meningitis with certain serotypes of EV is associated with particular signs: -hand-foot-mouth syndrome (EV71)(vesicular eruption); -nonspecific rashes (echovirus 9) The duration of illness is usually less than 1 week Laboratory findings low level of WBC count in CSF(100-1000/mm3); PMN may predominate early in the meningeal infection; Lymphocytic profile (>50%) over the first 8 to 48 hours; Glycorachia is normal or slightly decreased; CSF protein is normal or slightly elevated; Interferon- gama level is increased in viral meningitis; Isolation of virus from CSF, blood, throat, feces; Serology: neutralizing reaction and complement fixation PCR-based detection of EV. LYMPHOCYTIC CHORIOMENINGITIS VIRUS The virus is a member of the family Arenaviridae; it is transmitted by rodents (rats, mice). Infected persons could remain asymptomatic or present mild clinical manifestations; Less frequently severe neurologic disease could occur: meningoencephalitis, encephalitis. The course of meningitis is often prolonged. HERPES VIRUS MENINGITIS Aseptic meningitis may be produced by almost all the member of this family: Herpes simplex virus type1 and 2 (HSV-1 and HSV-2), varicellazoster virus, Epstein-Barr virus, cytomegalovirus (CMV), and human herpesvirus 6 (HHV-6). MOLLARET’S MENINGITIS Mollaret’s meningitis is a rare disease characterized by recurrent, benign episodes of aseptic meningitis, most commonly seen in young adults. Clinical manifestations Acute onset with fever, headache, nuchal rigidity; these symptoms resolve spontaneously within 2-5 days. Recurrent attacks appear weeks to months later. Laboratory diagnosis CSF exam reveal a mixed lymphocytic and PMN pleocytosis. An early finding are large, fragile, mononuclear cells (“endothelial cells” which are monocytes). After 24 hours Mollaret’s “endotelial” cells and PMN cells have disappeared. By 1 week CSF has completely normalized. Viruses of the herpesvirus family have now been strongly implicated in numerous cases; this association has potential therapeutic importance (acyclovir might prevent recurrences). Laboratory diagnosis of viral meningitis 1. CSF exam (presented above) 2. Cultures of the virus 3. Serological exams(become positive after 2-8 weeks from the onset) 4. Other tests: -leucogram ( leucopenia with lymphocytosis) -X-ray for paranasal sinuses -CT, MRI, funduloscopic exam for the exclusion of empyema, tumors, abscesses, etc. Differential diagnosis For the differential diagnosis of viral meningitis must be rule out other causes of aseptic meningitis syndrome: Bacteria: Leptospira species, Borrelia burgdorferi Mycobacterium tuberculosis Brucella species Mycoplasma hominis, pneumoniae Partially treated bacterial meningitis and other parameningeal infections (sinusitis, otomastoiditis). Fungi: Cryptococcus neoformans (ususlly in immunocompromised patients) Coccidioides imitis Histoplasma capsulatum Candida sp. Other Autoimmune disorders (systemic lupus erytematosus) Bechet’s syndrome is a systemic inflammatory disease characterized by: Aseptic meningitis, meningoencephalitis, seizures, cranial nerves palsies, hemiparesis (10-30%) Recurrent aphtous stomatitis: genital aphtae, uveitis, skin lesions (erytema nodosum, acneiform lesions). Treatment: glucocorticoid and immunosuppressive therapy Drugs(immunomodulators, antibiotics, AINS) Malignancies Kawasaki disease (aseptic meningitis develops in 25-70% of patients with Kawasaki disease) Vogt-Koyanagy-Harada syndrome TUBERCULOUS MENINGITIS Egidia Miftode Tuberculous meningitis (TB) is the most life-threatening form of tuberculosis. Pathogenesis . Following primary infection, there is a lymphatic and hematogenous dissemination to a multitude of sites, including the kidneys, bone marrow, liver, spleen, CNS The initial pathologic events in TB meningitis are secondary to a hypersensitivity recation to the bacilli within the subarachnoidian space. There is a resultant exudative inflammatory reaction; Exudative reaction can become extensive, and by consequence CSF absorption and circulation become impaired leading to progressive hydrocephalus; As hydrocephalus progress, the increase in intracranial pressure can lead to brain stem herniation; Vasculitis contribute to the neurologic sequelae. Clinical manifestations I. Stage I (first week-10 days) is characterized by nonspecific early signs (related to the increase in intracerebral pressure): emesis, decreased activity, lethargy, no neurologic deficits; II. Stage II (second week of evolution): signs of meningeal irritation, lethargy, minor neurologic deficits (cranial nerves palsies); III. Stage III (after three weeks of evolution ) seizures, severe neurologic deficits (pareses/paralysis), abnormal movement (chorea myoclonus, cerebellar ataxia), stupor or coma. Diagnostic tests CSF examination -clear CSF -several hundred WBCs, with the predominance of lymphocytes -low CSF chloride -increased level of protein concentration -low level of glucose( CSF/serum glucose ratio=20-25%) -acid fast staining with positive results -definitive diagnosis of TB meningitis depend on the growth of M. tuberculosis from CSF sample (that may require 4-6 weeks) -rapid diagnosis methods: adenosine deaminase level, mycobacterial antigen, mycobacterial antibody, PCR. Other diagnostic mesures Erytrocyte sedimentation rate is elevated -extraneural cultures: sputum, gastric culture, urine, lymph node, pleura, marrow and liver culture, flexible bronchoscopy with bronchoalveolar lavage -tuberculin test (almost 80% rate of positivity); malnutrition, debilitation and general immunosuppression could explain negative results; -funduloscopic exam; -chest X-ray -brain CT scan -MRI Complications -Hydrocephalus -Tuberculoma (can developed after 10 days until 9 years): papilledema and neurologic deficits. CT scan have demonstrated resolution of lesions as early as 12 weeks after the beginning of treatment. Sequelae Psychiatric sequelae: Neurologic sequelae. Endocrinologic abnormalities:, Mortality is near 20-30%. Prognostic factors corelated with poor prognosis are: advanced stages of the disease (two fold increase in mortality), extreme of youth or old age, coexistence of miliary disease, pregnancy or puerperium markedly elevated protein in CSF, spinal block, markedly decreased CSF glucose. Differential diagnosis I. Early in the disease: otitis media, gastroenteritis, influenza. II. CSF lymphocytic pleocytosis may be similar to that seen in: -viral meningoencephalitis (herpes simplex, etc) -fungal meningitis: cryptococcal meningitis, coccidiodomycosis. III. Brain abscess, malignancy, hemorrhage (see chapter encephalitis). IV. V. Chronic meningitis with infectious causes: Cryptococcus neoformans, Coccidioides immitis, Histoplasma capsulatum, Borrelia burgdorferi, Treponema palidum; Chronic meningitis with noninfectious causes: sarcoid, carcinoma, granulomatous angiitis, systemic lupus erytematosus, Behcet’s disease, Vogt-Koyanagi-Harada syndrome. Diagnosis 1. Fever, vomiting, progressive neurologic deterioration over several weeks 2. Family history of TB (in 70% of cases) 3. CSF lymphocytic pleocytosis, CSF/serum glucose reatio<0,25 4. Concurrent pulmonary TB (in 50% of cases) 5. Initial PPD >10mm induration (in 50% of cases) 6. Hydrocephalus(in infants). Treatment Table 1. Treatment with four antimycobacterial agents Drug First-line drugs Izoniazid Rifampin Pyrazinamide Ethambutol Streptomycin Second-line drugs Cycloserine Paraaminosalicylic acid (PAS) Kanamycin amikacin Ciprofloxacin Ethionamide Dose (mg//kg/day) Max. dose 10-20 mg/kg/day 10mg/kg/day 20-40mg/kg/day 15-25mg/kg/day 20-40mg/kg/day 300mg 600mg 2g 2,5g 1g 1-15 mg/kg/day 150 mg/kg/day 15 mg/kg/day 7,5 mg/kg/day 15-30 mg/kg/day 15 mg/kg/day 500mg 12 1g 1g 1,5g orally 1g Treatment must be continued: for 2 months with 4 drugs(first-line drugs). And for 7 months with rifampin+isoniazid Corticosteroids (Dexametasone or prednisone) are recommended if hydrocephalus, spinal block, cranial nerve palses, or coma is present. VIRAL ENCEPHALITIS Egidia Miftode Encephalitis is defined as an inflammation of parenchymal brain tissue clinically expressed by fever, consciousness impairment, seizures, focal neurologic deficits. Definitions Acute encephalitis is the brain inflammation that occurs over a relatively short period of time. Chronic encephalitis presents over weeks to months. Slow viral infections of the CNS (kuru, Bovine spongiforme encephalopathie) Classification upon the mechanism of the disease: 1. Primary encephalitis2. Postinfectious encephalitis -. Etiology Togaviridae-Alphavirus (arbovirus): Western, Eastern, Venezuelean encephalitis virus Flaviviridae-Flavivirus (arbovirus): -West Nile fever virus, -Japanese encephalitis virus -St. Louis encephalitis virus, -Tickborne encephalitis virus (TBE complex: louping ill, Powassan, TBE Western and Eastern subtype, Omsk hemorrhagic fever), -Murray Valley virus. Bunyaviridae (arbovirus) Herpesviridae: -Herpes simplex virus type 1 and 2 -Cytomegalovirus -Epstein –barr virus -Varicella –zoster virus -human herpes virus -6 Picornaviridae: echovirus Paramyxoviridae: mesles virus, mumps virus Orthomyxoviridae: influenza viruses Rhabdoviridae: rabies virus Retroviridae: HIV-1 Arenaviridae: lymphocytic choriomeningitis virus Adenoviridae: Adenovirus. Pathogenesis Enteroviruses and arboviruses have a viremic spread to the CNS. Rabies and HSV infection are prototypes of viral CNS infection. Poliovirus and reovirus infect CNS by viremia and neuronal spread. Clinical manifestations 1. General symptoms: fever, headache chills; 2. Symptoms of cortical involvement: trouble of consciousness (obtundation, somnolence, delirium, confusion, coma), seizures, motor deficit, sleepness, dysphasia; 3. Symptoms of cerebral peduncles involvement: paresis of cranial nerves, respiratory and cardiovascular dysfunction, hemiparesis; 4. Spinal symptoms: paraparesis and tetraparesis; 5. Meningeal signs and symptoms Laboratory features CSF: Glucose level is normal/slightly elevated Protein level is moderately raised Pleocytosis: usually <500/mm3, with a predominance of mononuclear cells (early in the disease could be as high as 1000/mm3, with a predominance of neutrophils); persists for 3-6 weeks (may lasts for as long as 3-4 months). Specific diagnosis Viral culture or antigen detection in brain Serologic titers: hemagglutination inhibition (HI), complement fixation (CF), neutralizing antibody titer (NA), immunofluorescent antibody (IFA), counterimmunoelectrophoresis (CIE), CSF IgM (ELISA) PCR Tick-borne encephalitis The Western subtype virus produces a biphasic febrile illness: First phase lasts 2-7 days without signs/symptoms of meningoencephalitis. An afebrile and relatively asymptomatic period lasts 2-10 days Second phase (in 1/3 of patients): fever and symptoms of meningitis and meningoencephalitis. The Eastern subtype virus is characterized by a monophasic course: flushing of the face and neck, conjunctival injection, headache, somnolence, nausea, vomiting, dizziness, myalgia, hyperestesia. The main clinical neurological syndromes associated with TBE are: A. Febrile headache, with myalgia,. B. Aseptic meningitis: C. Meningoencephalitis: y. D. Meningoencephalomyelitis: Differential diagnosis Infection with other tick-borne pathogens: tularemia, spotted fever group of rickettsioses, babesiosis; Other febrile headache: infectious mononucleosis, influenza, typhoid fever, shigellosis, legionellosis, relapsing fever, erlichiosis, brucellosis; Infections causing meningitis meningoencephalitis or encephalomyelitis: Syndromes mediated by bacterial toxins: botulism, tetanus, Bordetella pertussis infection Noninfectious encephalopathies: Neoplastic lesions Guillain – Barre syndrome is an acute inflammatory demyelinating polyneuropathy Behcet’s disease Vogt-Koyanagi-Harada syndrome is an autoimmune disease: -Meningitis: Severe anterior and posterior uveitis -Vitiligo, alopecia -Dysacousia and tinnitus Treatment There is no curative therapy for TBE. Treatment is supportive (antiedematous drugs, parenteral hydration, symptomatics). Bed rest for up to 2 weeks improves outcome. In severe cases corticosteroids could be administrated. Herpes Simplex virus encephalitis Decreasing level of consciousness with focal neurologic findings Sometimes presence of skin vesicles CSF pleocytosis and proteinosis The absence of bacterial and fungal pathogens in CSF Focal electroencephalographic (focal slowing, spiking, and paroxysmal lateralizing epileptiform discharges), CT and MRI findings. Treatment Acyclovir - at a dose of 10mg/kg every 8 hours (30mg/kg/day), for a period of 10-14 days. West Nile encephalitis The disease incidence was 12.4 per 100.000 inhabitants. Epidemiology The virus multiplies in various species of birds, some of which get severely sick, like humans, whereas others harbor the virus without signs of disease. Humans, and occasionally cats and horses, get infected Clinical manifestations After an incubation period of 3 to 6 days, disease starts without any prodromal stage with: - fever, joint pains and headache; - temporary skin rash - lymph node swelling. -a typical biphasic fever progression is observed with West Nile virus: after a few days of normal body temperature, fever increases again strongly. The diagnosis detection of specific antibodies and/or the detection of viral RNA by PCR. The lethality of this form of disease is between 3 and 15%. POLIOMYELITIS Egidia Miftode Poliomyelitis is a clinicopathologic syndrome characterized by transient or permanent paresis of one or more extremities and aseptic meningitis. Etiology Polioviruses belong to the genus Enterovirus (family Picornaviridae). Three polioviruses serotypes are recognized: type 1-Brunhilde, type 2Lansing and type 3-Leon. - single –stranded RNA genome approximately 7500 bases in length. Epidemiology The possible routes for polioviruses transmission are: Fecal-oral transmission is the most important route Spread through upper respiratory tract secretions are favored by crowding, water quality, levels of hygiene Risk factors Recent intramuscular injections Injuries Exercise during the early stages of the major illness. Pathogenesis After ingestion, polioviruses implant in the oropharynx and small bowel (Payer’s patches). “Minor” viremia occurs as a consequence of spread to the regional lymph nodes and to bone marrow, liver and spleen. -“major” viremia, which coincides with the onset of the clinical symptoms associated with the “minor” illness 3 to 7 days after infection. Immunity to poliovirus infection is type-specific. . Clinical manifestations Approximately 95% of poliovirus infections are inapparent. Acute clinical poliomyelitis has 2 phases (” dromedary “ pattern): Minor illness (coinciding with viremia); has a duration of 1-5 days -incubation period is 3-7 days -consists of nonspecific symptoms: -frequently resolves within 1-2 days B. Asymptomatic period: 3-5 days C. Major illness -incubation period is 9-12 days Nonparalytic poliomyelitis: -abrupt onset of headache, vomiting, meningeal signs -CSF pleocytosis at an early stage,; -hypotonia Paralytic stage (within 2-3 days): -fever -prodromal myalgias; -paresis, paralysis (asymmetric, flaccid, involves proximal muscles of the limbs), progress for 1-7 days after onset; -deep tendon reflexes become absent; -cranial nerve involvement (the 9-th and the 10-th are the most commonly involved). Evolution Recovery of patients with limb paresis takes weeks to months after acute disease. Paralysis improves slowly as follows: 60% of eventual recovery is achived by 3 months and 80% by 6 months. Minimal further improvements continue over almost 2 years. Classification: A. Nonparalytic poliomyelitis: “minor” illness, meningitis (normal value of proteins in CSF in the early stage, and increased level of proteins after 2 weeks of evolution) B. Paralytic poliomyelitis: spinal forms, “bulbar poliomyelitis” Meningomyeloencephalitis: C. Complications 1. 2. 3. 4. 5. Respiratory: Cardiovascular:; Gastrointestinal: paralytic ileus, gastric dilatation; Urinary: ileus of the bladder, urinary tract infection; Decubitus ulcers Laboratory findings Virus can be grown in tissue culture from pharyngeal swab, (in the first week of the illness), from the feces (for at least 3 weeks), from blood and CSF (less frequently). Serological tests: complement fixation reaction and neutralizing reaction (neutralized antibodies are life-long persistent). Differential diagnosis Paralytic diseases caused by nonpolio enteroviruses; Guillain-Barre syndrome: paralysis is classically ascending, symmetric, and accompanied by sensory abnormalities. Other conditions with acute paralysis: transverse myelitis, botulism, encephalitis, epidural abscess, intramedullary abscess, cord tumors, brucellosis, syphilis. Prognosis Nonparalitic poliomyelitis has a complete recovery, and only 50-55% of paralysis has the same good evolution. Overall mortality for spinal pliomyelitis is about 4%-6% and for bulbar poliomyelitis is 25-75%. Treatment Treatment consists of supportive therapy and reduced physical activity for 710 days in nonparalitic poliomyelitis and for 10-14 days in paralitic forms. Mechanical ventilation is sometimes required in severe cases. Prevention 1. Live, attenuated poliovaccine (OPV) 2. Inactivated poliovirus vaccine (IPV). ENTEROVIRUSES INFECTIONS Daniela Leca As members of the genus Enterovirus, the group A coxsackieviruses, group B coxsackieviruses, echoviruses, and newer enteroviruses Epidemiology The enteroviruses have a worldwide distribution. The proportion of infected individuals who will develop illness varies from 2 to 100 percent Pathogenesis and pathology After primary replication in the epithelial cells and lymphoid tissues in the upper respiratory and gastrointestinal tracts, viremic spread to other sites can occur. Illnesses caused by Enteroviruses Acute Aseptic Meningitis Acute aseptic meningitis is a syndrome characterized by signs and symptoms of meningeal irritation and cerebrospinal fluid (CSF) pleocytosis in the absence of bacteria or fungi. Encephalitis Frank encephalitis is an unusual manifestation of CNS infection with coxsackieviruses and echoviruses that sometimes complicate the course of aseptic meningitis. The differential diagnosis includes: encephalitis due to other viruses (arboviruses, herpes simplex virus -especially in focal encephalitis, mumps); postinfectious encephalitis after measles, rubella, varicella, or pertussis; Reye’s syndrome; Lyme disease; toxic encephalopathies. Paralysis and other neurologic abnormalities Sporadic cases of flaccid motor paralysis have been associated with several coxsackievirus (A7, A9, B1-5) and echovirus (6,9) serotypes, and with enterovirus 71. Guillain-Barré syndrome is caused by coxsackievirus serotypes A2, A5, and A9, and with echovirus serotypes 6 and 22. Systemic coxsackievirus B2 disease Reye syndrome. Exanthems Coxsackieviruses and echoviruses cause a variety of exanthems: rubelliform or morbilliform, roseoliform, vesicular, and petechial. Roseoliform exanthems. Exanthem subitum (roseola infantium). Hand-foot-and-mouth (HFM) disease or vesicular stomatitis. Generalized vesicular eruptions are caused by coxsackievirus A9 and echovirus 11. Petechial and purpuric rashes Acute Respiratory Disease Undifferentiated febrile illnesses ("summer grippe"). Coxsackieviruses A21 and A24 produce illness resembling the common cold. Coxsackieviruses B have been associated with a variety of respiratory illnesses,. Herpangina Herpangina primarily affects children between the ages of 3 and 10 years. Coxsackieviruses A (types 1–10, 16, 22) are the etiologic agents in most cases. The illness begins with fever of 37.7–40.5 0C. Sore throat and pain on swallowing precede the appearance of the enanthem that begins as punctate macules,. Epidemic Pleurodynia Epidemic pleurodynia is an acute infectious disease characterized by fever and sharp, spasmodic pain in the chest or upper abdomen. Pain in the chest may mimic pneumonia, pulmonary infarction, myocardial ischemia, and the preeruptive phase of zoster. Myopericarditis Enteroviral myocarditis occurs at all ages but has a special predilection for adolescents and active young adults Most children and adults recover uneventfully. The complications consist in persistent electrocardiographic abnormalities, cardiomegaly, chronic congestive heart failure. Chronic constrictive pericarditis occurs after 5 weeks to 1 year. Differential diagnosis include: others viral myopericarditis Coxsackievirus and Echovirus Disease in the newborn Infant Many enterovirus serotypes causes the same self-limited clinical syndromes in neonates as they do in older persons (e.g., aseptic meningitis, exanthems, hand-foot-and-mouth syndrome), but some serotypes are capable of producing fulminant, frequently fatal disease (group B coxsackievirus serotypes 2–5 and echovirus 11). Acute hemorrhagic conjunctivitis is a contagious ocular infection characterized by pain, swelling of the eyelids, and subconjunctival hemorrhages . Laboratory diagnosis The diagnosis is established by: virus isolation from throat, swabs, stool or rectal swabs, body fluids, and occasionally tissues; polymerase chain reaction, enhancing and speeding detection in tissue and CSF, but is not currentlly available serodiagnosis -; White blood cell counts and erythrocyte sedimentation rates are usually mildly elevated; Hyperbilirubinemia and elevated transaminase and alkaline phosphatase levels. Prophylaxis and treatment Glucocorticoids are contraindicated. GASTROINTESTINAL INFECTIONS Dr. E Miftode Etiology The microorganisms that cause infectious gastroenteritis are: Bacteria - Shigella - E. coli (enteroinvasive, enterohemorrhagic E.coli, entero toxigen E. coli) - Salmonella spp - Vibrio cholerae - Vibrio parahaemolyticus - Clostridium difficile - Clostridium perfringens - Campylobacter jejuni - S. aureus - Bacillus cereus - Yersinia enterocolitica Viruses - Rotavirus - Astrovirus - Caliciviruses - Adenoviruses - Parvoviruses (Norwalk,Hawaii, agents, etc) - Coronaviruses Parasites - Entamoeba histolytica - Giardia lamblia - Cryptosporidium Pathogenesis There are three types of enteric infections: Type I - is a noninflammatory (enterotoxin or adherence/superficial invasion) mechanism Etiology of this type of diarrhea: - S. aureus - Vibrio cholerae - Bacillus cereus - E. coli (ETEC-entero toxigen E. coli) - Clostridium perfringens - Rotavirus Norwalk-like viruses - Giardia lamblia - Cryptosporidium Type II. Characterized by inflammatory destruction of the ileal or colonic mucosa (by invasion, cytotoxin), producing dysentery illness. Stool examination reveal fecal polymorphonuclear leukocytes and a high level of lactoferrin. Etiology: - Shigella - E. coli (enteroinvasive and enterohemorrhagic E.coli) - Salmonella enteritidis - Vibrio parahaemolyticus - Clostridium difficile - Campylobacter jejuni - Entamoeba histolytica Type III. Characterized by penetration through an intact mucosa to the reticuloendothelial system. Stool examination reveals fecal mononuclear leukocytes. Etiology: - Salmonella typhi - Yersinia enterocolitica The intestinal host factors that may help prevent the acquisition of diarrheal disease are: 1. Host species, genotype and age 2. Personal hygiene.. 3. Gastric acidity4. Intestinal motility, 5. -Normal bacterial flora 6. Intestinal immunity is represented by phagocytic, humoral and cellmediated elements.. 7. Human milk is protective. Microbial factors involved in producing disease are: 1. Toxins: a. Neurotoxins:(Clostridium botulinum, S. aureus, B. cereus) b. Enterotoxins (V. cholerae, E. coli, Salmonella, Shigella dysenteriae, B. cereus) c. Cytotoxins (Shigella, C. perfringens, V. parahemolyticus, S. aureus, EHEC, Helycobacter pylori, Campylobacter jejuni) 2. Attachment 3. Ivasiveness 4. Other virulence factors: motility, chemotaxis, mucinase production General aspects and diagnosis Viral diarhea The principal presentation of rotavirus infection is acute voluminous watery diarrhea with or without vomiting Diagnosis can be made by electron microscopy, antigen detection or genome detection (RT-PCR)-the latter is the most sensitive and can also be adapted to genotype of the virus. The human astrovirusis are the second commonest cause of diarrhea in hospitalized children. The clinical features are similar to these of rotavirus but in general are less severe. The human Caliciviridae have the typical “star of David” morphology and small round-structured viruses. Epidemic and sporadic diarrhea and vomiting can result from calicivirus infection. Diagnosis has improved greatly with the application of antigen detection and RT-PCR. ACUTE DISENTERY Egidia Miftode Shigella spp. are the main cause of dysentery worldwide and have also been related to hemolytic uremic syndrome. Dysentery is defined as frequent, small bowel movements accompanied by blood and mucus with tenesmus or pain on defecation. There is a substantial risk of person-to person transmission. . Pathogenesis Invasion of Shigella in the colon produces an inflammatory colitis. Clinical manifestations The incubation period ranged from 6 hours to 9 days (usually <72) The onset is manifested by fever, followed by small amounts of watery and then bloody diarrhea with mucus. Patients present abdominal pain, tenesmus and pain on defecation. Extraintestinal manifestations. Complications Hemolytic-uremic syndrome Pseudomembranous colitis Arthritis (10%) - 2 to 5 weeks after the dysenteric illness. Rectal prolapse Reiter syndrome (arthritis, urethritis and conjuntivitis) Toxic megacolon Protein-loosing enteropathy. Differential diagnosis I. Specific infectious processes: II. Proctitis: III. Other syndromes: - Necrotizing enterocolitis - Enteritis necroticans - Pseudomembranous enterocolitis (Clostridium difficile) - Diverticulitis - Typlitis IV. Heavy metal poisoning (As, Sn, Fe, Cd, Hg, Pb) V. Syndromes without known infectious causes: - Idiopathic ulcerative colitis - Chron’s disease - Ischemic colitis - Radiation enteritis. VI. Noninfectious endocrine causes: VII. Impaired small bowel absorption: tropical sprue, enzymes deficiencies, solute loads. VIII. Noninfectious chronic noninflammatory diarrhea: IX Tumors, polyposis Principles of treatment in infectious diarrhea Treatment consists of: Correcting and maintaining hydration Eliminating the etiological agent if possible Maintaining adequate nutrition Rehydration therapy Clinical findings to estimate fluid volume depletion in diarrhea Findings Central pulse Peripheral pulse Skin turgor Eyes Muscles Appearance Urine flow Depletion (percentage of body weight) 0-5 5-10 10-15 Full Full Weak “ Weak Absent N Decreased Poor N Slightly sunken Sunken N Some cramps Severe cramps Alert/thirst Alert/thirsty Restless/+thirsty N reduced absent A. Replacement of fluid loss Deficit of body weight <5% 5-10% 10-15% Volume of fluids 50 ml/kg 50-100 ml/kg 100-150 ml/kg B. Supply of metabolic necessities Body weight Volume of fluids 1-10 kg 100 ml/kg 11-20 kg 1000 ml+(50 ml/kg x nr of kg>10 kg) 21-80 kg 1500 ml+(20 ml/kg x nr of kg > 20 kg) Composition of oral electrolyte solutions in Who solution (1 packet) is as follows: 3,5 g NaCl, 1,5 g K, 2,9 G trisodium citrate dihydrate and 20 g of glucose. The solution is made by addign 1 packet of electrolyte to one liter of water. FOOD BORNE DISEASES Dr. Egidia Miftode, Dr. C. Corcaci Food borne disease is defined as an acute illness with gastrointestinal or neurologic manifestations that results from ingestion of contaminated foods (with microorganisms or microbial toxins). Table 1. Etiology and pathogenic mechanism of food borne diseases Preformed toxin Toxin produced in vivo S. aureus Bacillus cereus C. botulinum C. perfringens Bacillus cereus ETEC V. cholerae Shiga-toxinproducing E. coli C. botulinum (in Tissue invasion EIEC Salmonella Shigella Campylobacter jejunii Toxin production and/or tissue invasion V. parahemoliticus Yersinia enterocolitica infants) Staphylococcal food poisoning – occurs after consumption of ham, milk, eggs, salads, poultry. Characteristics: Short incubation period (1-6 hours) Manifested by nausea and vomiting (rarely diarrhea) Fever is uncommon Enterotoxins produced by S. aureus are heat-stable protein (A-E) B. cereus Occurs after ingestion of fried rice, meats, vegetables C. perfringens Food borne disease occurs after ingestion of meat (beef and poultry), gravies. E. coli Enteroaggregative E. coli (EAEC), Enterohemorrhagic (EHEC), Enteropathogenic(EPEC), Enterotoxigenic(ETEC). E. coli remains the most common bacterial enteric pathogen reported worldwide. Enterohemorragic E. coli and especially strain O157:H7 has emerged as a cause of serious gastrointestinal disease since its recognition 20 years ago. Clinical manifestations: fever, abdominal cramps and diarrhea bloody diarrhea without fever-when Shiga toxin-producing strains of E. coli are involved. complications are: hemolytic –uremic syndrome, thrombotic thrombocytopenic purpura. Salmonella Food poisoning occurs after comsumption of: poultry, eggs, beef, dairy products, fresh produce (tomatoes, melons). Clinical manifestations (frequently within 16-48 hours): fever, abdominal cramps, watery diarrhea. Yersinia enterocolitica Yersinia enterocolitica serogroup O:3 is the predominant strain in acute enterocolitis, primarily affecting children younger than 5 years of age. In young children the disease is a febrile diarrhea. In older children and in adults Y. enterocolitica mimics acute appendicitis. Yersinia enterocolitica septicemia is less common and is most often reported in patients with diabetes mellitus, severe anemia, cirrhosis, malignancy and in elderly patients. Erythema nodosum and reactive polyarthritis Vibrio parahaemolyticus V. parahaemolyticus is a gram-negative, halophilic,marine organism that causes a self-limited gastroenteritis. Diarrhea, cramps, weakness, chills, headache, fever and vomiting Campylobacter jejuni Campylobacter spp.-the cytolethal distending toxin of Diagnosis: fever, abdominal cramps, diarrhea within 16-72 hours after the ingestion; fecal blood, and polymorphonuclear leukocytes. Duration of illness is < 1 week with relapse in untreated patients. Complications: Guillain-Barre syndrome occurs 1-3 weeks after diarrheal illness. Treatment: erythromycin. Differential diagnosis 1. Heavy metal food borne disease (Copper, zinc, Cadmiu) 2. Fish poisoning evolves with symptoms resembling those of a histamine reaction: 3. Mushroom poisoning (onset within 2 hours). Laboratory diagnosis Culture of different specimens: feces, vomitus, serum, blood and food handlers Isolation and serotyping of organism from the feces:. Rectal swab Toxin testing of food Serology for Y. enterocolitica, V cholerae, Shiga toxin producing E. coli. Therapy Antibiotics should be avoided in uncomplicated gastrointestinal infections caused by non-typhoidal Salmonella, C. perfringens, B. cereus or staphylococcal food poisoning. Doxycycline and TMP-SMZ have been shown to reduce the incidence of traveler’s diarrhea-is not recommended universal use of doxycycline. TYPHOID FEVER Dr. Egidia Miftode Typhoid fever is caused by Salmonella typhi, but, other Salmonellae (S. paratyphi, S. schottmulleri, S. hirschfeldii, and S. choleraesuis) may cause similar clinical syndrome. Etiology S. typhi, a gram-negative rod, possesses two O antigens (surface polysaccharide) and one H (flagellar) antigen; the envelope antigen, Vi (virulence antigen) is not constant. Pathogenesis S. typhi are ingested with food/water pass from the stomach into the duodenum. In the distal small bowel they penetrate the intestinal epithelium, over Payer’s patches. They multiply in the intestinal lymphoid tissue and then disseminate via lymphatic circulation or hematogenous route. Local concentration of endotoxin are responsible for cytotoxic and ischemic damage, and circulating endotoxin is responsible of septic shock. Inflammation of the epithelial cells and the subsequent release of chemical mediators, which can stimulate the secretion of fluid into the gut lumen may explain the diarrhea. Clinical manifestations Incubation period is 7-14 days. Clinical features are: 1. Fever: begins as a remittent feverduring the first 72 hours, rising in a stepwise fashion after this period associated with chills. 2. 3. 4. 5. 6. Diarrhea is present in 40-50% of cases, and, sometimes constipation. Headache Diffuse or localized abdominal pain (often in the right upper quadrant) Relative bradicardia Rose spots: maculopapular lesions on the abdominal wall and chest (in the first few days of illness). 7. Splenomegaly 8. Pharingitis is infrequent. Laboratory findings For definitive diagnosis 1. Isolation of S. typhi/another Salmonella spp from: Blood (positive for about 2 weeks in untreated patients) Bone marrow Stool (positive for several months in untreated patients) Urine Duodenal contents 2. Serology Widal test-detect anti-S.typhi antibodies ELISA using a cell envelope antigen or lipopolysaccharide of S. typhi or purified Vi antigen. PCR. Additional laboratory tests: - leukopenia - absence of eosinophils on peripheral smears - mildly elevated bilirubin/transaminase level - urinalysis: proteinuria, piuria, casts. - Chest radiographic films reveale infiltrate - Mononuclear cells in fresh stool specimens. Differential diagnosis 1. Enteric fever-like syndrome causing by other bacteria : Yersinia enterocolitica, Y. pseudotuberculosis, Campylobacter (manifested by fever, headache, abdominal pain). 2. Systemic infection that may mimic typhoid fever: Bacterial infections: Parasitic infections: Viral infections:. Mycotic infections: disseminated histoplasmosis. Rickettsial infections: epidemic/endemic typhus, Q fever, erlichiosis, Rocky Mountain spotted fever. Complications Major complications are: - hemorrhage from ulcerating lesions –late in the course of disease (2% of untreated patients) - perforations of the terminal ileum or proximal colon. Other complications are: psychosis, cholecystitis, hepatitis, meningitis, pericarditis, pneumonitis, nephritis. Treatment Antibiotic therapy can eliminate the fever over 3 to 5 days. 1. Ampicillin: 2. Chloramphenicol3. Trimethoprim-sulfamethoxazole; 4. Fluoroquinolones: ciprofloxacin and ofloxacine 5. Third generation cephalosporins; 6. Monobactam. Evolution Untreated patients maintain a constant temperature pattern for 3 to 4 weeks. Relapses occur in about 10-15% of treated patients, usually about 2 weeks after the therapy was stopped. These patients need another short course of therapy. Prevention: Typhoid vaccine is recommended for travelers in endemic areas where the water supply is suspect. BOTULISM Dr. Egidia Miftode Botulism is a paralytic illness caused by the neurotoxin produced by the bacterium C. botulinum. Etiology Members of genus Clostridium are gram-positive, anaerobic bacilli. C. botulinum and closely related organisms produce toxins designated as types A, B, C, D, E, F, and G. The toxins causing this disease are among the most potent bioactive substances known (the oral lethal dose for humans is 0.05-0,5 microg). Pathogenesis There are four clinical type of botulism Foodborne botulismWound botulism Infant. “Infectious“ botulismClinical manifestations The latent period is typically 12-36 hours, but can range from 6 hours to 10 days. Patients are afebrile in the absence of complications. The symptoms are: gastrointestinal-nausea, vomiting, abdominal cramps, diarrhea/constipation, dryness of mucous membranes; neurologic, which are manifestations of cranial nerve dysfunction: muscle weakness; manifestations of parasympathetic nervous system dysfunction: pupils may be dilated or nonreactive. Complications include otitis media, aspiration pneumonia, respiratory distress syndrome. Laboratory diagnosis Detection and identification of botulinum toxin in blood, feces and in the food consumed before onset of illness. Isolation of C. botulinum from the stool. CSF is normal Differential diagnosis The diseases that may be confused with botulism are: tick-borne encephalitis, diphtheria, poliomyelitis, Guillain-Barre syndrome, myastenia gravis, food poisoning of other etiology. For infant botulism differential diagnosis includes: sepsis, dehydration, pneumonia, Epstein-Barr infection, diphtheria, congenital myastenia gravis, muscular dystrophy, hypothyroidism, metabolic and toxic conditions which may produce weakness and hypotonia. Mortality is 25-30%. Therapy 1. Supportive care, nutritional support; 2. Prevention of nosocomial pulmonary and urinary tract infection. 3. Trivalent botulinum antitoxin (neutralize only circulating toxin)-must be administrated as early as possible, with a test for the hypersensitivity. Antitoxin must be given intravenously, in one vial (if there are no signs of hypersensitivity). Hypersensitivity reactions must be averted through the use of human botulism immune globulin. Botulism antitoxin desensitization: with serial subcutaneous injections of antitoxin in 20-minutes intervals . 4. Guanidine hydrochloride 5. High-dose penicillin therapy is generally given 6. Antibiotics if there are complications 7. Debridement of the wound C. botulinum spores are not killed by boiling at 100 C. The toxin is heatlabile, so it may be inactivated by boiling of foods before consumption. Mortality is 25-30%. CHOLERA Daniela Leca An acute infection involving the entire small bowel, characterized by profuse watery diarrhea, vomiting, muscular crumps, dehydration, oliguria and collapse. Etiology V. cholerae is not invasive, short (1.5-3 mm by 0.5 mm), curved, motile, gram negative, aerobic rod, oxidase-positive, grows luxuriantly in alkaline media in the presence of bile salts, and produces a neuraminidase that has the property of degrading gangliosides to the monosialosyl form, which is the specific receptor for cholera toxin. Antigenic structure include: - flagellar (H) antigens; antisera prepared against them do not distinguish the vibrio causing human epidemic disease from water vibrios. - somatic (O) antigens do distinguish V. cholerae Ogawa, Inaba, and Pathophysiology V. cholerae is swallowed either with water or food. It must survive passage through the stomach to colonize the small intestine even though it is extremely acid sensitive. Epidemiology Cholera is spread by ingestion of water, sea foods and other foods contaminated by the excrement of persons with symptomatic or asymptomatic infection. Clinical manifestations Cholera may be present: in an asymptomatic state, mild diarrhea, or the typical "full-blown" syndrome. The first symptoms of cholera are an increase in peristalsis. There is little abdominal pain in cholera, most of the anxiety, muscle cramps, thirst, and faintness being related in their prominence to the rate of fluid loss. Rarely, ileus may occur at the onset of illness with profound -"Cholera Sicca". Altered consciousness. Electrolyte Uncomplicated cholera is self- limited; recovery occurs within 3 to 6 days. The fatality rate can be > 50% in untreated severe cases but is < 1% with prompt and adequate fluid and electrolyte therapy. Diagnosis Clinical diagnosis rests on the history of acute onset and the watery stool in the absence of high fever or much abdominal pain. Laboratory diagnosis 1. The stool, microscopically, will show limited numbers of white cells and rarely red cells. 2. darkfield or phase microscopy,. 3. Cultures 4. Antibodies to the specific somatic O antigens: direct agglutination of heated V. cholerae, the agglutination of chicken red blood cells that have been coated with antigens, a vibriocidal test Treatment . The water and salts lost in the cholera stool must be replaced in comparable amounts and concentrations. The replacement can, except in severe cases, be accomplished by mouth (see chapter “Diarrheal diseases”). Intravenous replacement therapy is needed when: the volume of stool output exceeds 100 ml/kg/24 hour, appropriate oral rehydration has not been given Antimicrobials will shorten the duration of diarrhea and, thereby, reduce fluid losses in cholera. These includes: 1. Tetracycline: 2. Ampicillin, 3. Trimethoprim-sulfamethoxazole 4. Furazolidone:, 5. doxycycline:) 6. fluoroquinolones: 7. The most important reversible life-threatening complication of cholera after fluid and electrolyte loss is hypoglycemia.. Prevention Prompt prophylaxis with tetracycline 500 mg orally q 6 h in adults (12 mg/kg q 6 h in children < 8 years of age TRICHINOSIS Egidia Miftode Trichinella spiralis was first seen in human tissue at autopsy in the early 1800s. By the 1900s, trichinosis was definitely recognized as a public health problem. Table 1. Trichinella spiralis: life cycle stages Time after infection first 2-4 hours 30 h Day 6 Day 10 Day 14 Day 21 Day 23 Month 1 Stage in life cycle Excysted larvae enter intestinal mucosa Worms mature and mate Females deposit larvae/muscle, invasion begins Heaviest muscle invasion Decrease in larval deposition Encapsulation of larvae Intestine free of adult worms Encapsulation almost complete Month 3 Month 6 Year 1 Year 6 Adult worms die Cyst calcification begins Cyst calcification usually complete Most larvae still viable within calcified cyst Clinical disease Incubation has a 1-30 days duration I. Intestinal effects. II. Muscle penetration and larva encapsulation (invasion phase) – fever, facial (periorbital) edema, muscle pain, swelling, and weakness; conjunctivitis, subconjunctival hemorrhages, pruritus. Skin rashes include: Less frequent manifestations are: Myocarditis Central nervous system involvement (10-20%): Pneumonitis: pulmonary infiltrates, pleural reaction III. Convalescent phase (after 2 months): fever subsides, improvement in muscular symptoms. Laboratory abnormalities A. Peripheral eosinophilia (of at least 20%, and possibly up to 90%) is present during the muscle invasion phase of the infection. B. Leukocytosis C. Hypoproteinemia D. Hypokalemia E. Elevated cretinphosphokinase F. Mild elevation of alaninaminotransferase level (ALAT) Diagnosis 1. The history may suggest possible trichinosis (consumption of rare or raw infected meat). 2. Eosinophilia 3. Serologic tests: 4. Muscle biopsy (gastrocnemius, deltoid, and biceps)- does not provide positive results until 2 to 3 weeks after the onset of the illness. 5. Larvae or adult worms are rarely recovered during the intestinal phase (diarrhea). 6. Examination of suspect meat may reveal larvae (artificial digestion procedure). Differential diagnosis During the intestinal phase: Viral gastroenteritis, Food poisoning During the visceral phase: Typhoid fever Dermatomiositis, Serum sickness Polyarteritis nodosa, Periorbital cellulitis, angioneurotic edema Rheumatic fever Trypanosomiasis Neurologic involvement may mimic: polyneuritis, acute anterior poliomyelitis, myastenia gravis, meningitis, viral encephalitis Cardiac involvement may mimic: viral or bacterial myocarditis, endocarditis, ischemic cardiomyopathy. Tretment Albendazole: 400mg po bid, 14 days. Mebendazole: 5mg /kg po bid, 10-13 days. Concomitant prednisone: 40-60 mg po qd; it is indicated in severe disease VIRAL HEPATITIS Egidia Miftode Table 1. Characteristics of hepatitis viruses Property Family Nucleic acid type Transmission Maximum titer Fulminant disease Chronicity Malignancy Serologic tests Antigen Antibody HAV Picornaviridae RNA HEV ? RNA HBV Hepadnaviridae DNA HCV Flaviviridae RNA Fecal-oral 109/ml Rare Parenteral-sexual 109-10/ml Rare Parenteral 106/ml Rare No No Fecal-oral ? Pregnant women No No 1-10% Yes 50-90% Yes Yes Yes Yes Yes Yes Yes No Yes Genotypes 7 3 5 Vaccination Yes No Yes >6weeks Types 1-6 with subtypes a, b, c No HEPATITIS A Hepatitis A virus (HAV) is a member of picornaviridae family (which includes poliovirus and rhinoviruses, agents of the common cold). The virus particle is of 27nm in diameter is not enveloped has an outer capsid which surrounds a single-stranded RNA. Risk factors for acquisition: contact with acutely ill individuals travel to endemic areas homosexual activity contact with children attending day care centers illicit drug use Among the persons under 18 years old, 50% in France, 90% in Portugal and >95% in Romania present antibodies against HAV. Pathogenesis During acute HAV infection, there is an initial viremia and fecal shedding of viruses; viremia persists for up to 3 weeks. Histopathological aspects include the existence of: hepatocellular necrosis centrilobular cholestasis periportal infiltration of the liver with mononuclear inflammatory cells Clinical manifestations Only 10% of A-hepatitis are clinically manifested. The incubation period: the symptoms of HA usually appear about 4 weeks after exposure, but the interval may range from 2-6wks. The clinical manifestations are different in adults and young children (children of less than 2 years old experience asymptomatic infection or develop other symptoms than those suggestive for hepatic inflammation. A. Prodromal (preicteric) phase lasts for 4-10 days could be very variable: 1. Gastrointestinal symptoms: anorexia, nausea and vomiting, right upper-quadrant pain, 2. diarrhea (in children –20% of cases ), distaste for cigarettes (in smokers only); 3. Influenza-like - syndrome: fever /chills, muscle pain, headache; 4. Rheumatic syndrome: arthralgias (usually disappear after onset of jaundice); 5. Eruptions: petechiae, measles/scarlet fever-like exanthema; 6. Fatigability; 7. Pruritus; 8. Jaundice 9. Acute abdomen (mimicking an appendicitis) B. Icteric phase – is ushered by the appearance of dark, golden-brown urine due to bilirubinuria, followed one to several days later by pale stools yellowish coloration of the mucous membranes, conjunctivae, sclerae and skin. The physical examination 2 special forms of evolution may be encountered: Cholestatic hepatitis A - >12 weeks of jaundice Relapsing hepatitis A Diagnostic 1. AST (Aspartate Aminotransferase) or SGOT (Serum Glutamic-Oxalacetic Transaminase) (N = 5 – 54 U/L). 2. ALT (Alanine Aminotransferase) or SGPT (Serum Glutamic-Piruvic Transaminase): (N = 0 – 36 U/L). 3. Alkaline Phosphatase (N = 40 –120 U/L). 4. GGT (Gamma Glutamyltransferase) (N = 3 – 59 U/L). 5. LDH (Lactose DeHydrogenase) (N = 135 – 225 U/L).. 6. Total bilirubin 7. Direct bilirubin (conjugated) (N = 0 – 5 μmol/L). 8. Albumin (N = 35 – 50 g/L)9. PT (Prothrombin Coagulation Time) (N = 10 – 12 sec.) or QUICK Index (N≥80%). 10. Urine tests: bilirubin appears in the urine in the prodromal phase and may lead to the diagnosis 11. Peripheral blood: 12. Serological confirmation is always required, by the specific detection of IgM anti HAV. Complications Neurological complication: encephalitis, meningoencephalitis, GuillainBarre syndrome, mononeuritis affecting cranial or peripheral nerves. Myocardial involvement: myocarditis and electrocardiographic changes Pancreatitis Pleural effusions and ascites Aplastic anemia Differential diagnosis of jaundise I Abnormalities of bilirubin metabolism 1. Increased bilirubin production (unconjugated bilirubinemia): Hemolysis Ineffective erythropoiesis: megaloblastic anemias, myeloproliferative diseases, protoporphyria etc Blood transfusions Resorbtion of haematomas 2. Decreased hepatic clearence: a. decreased uptake and/or conjugation of bilirubin - Gilbert syndrome, Crigler Najjar syndrome - Rifampin - Physiologic jaundice of neonate b. Decreased canalicular excretion of bilirubin: Dubin-Johnson and Rotor syndromes II. Hepatocellular jaundice 1. Acute/subacute hepatocellular damage Acute hepatitis: viral (CMV, Epstein-Barr virus, herpes), bacterial (leptospirosis, brucellosis, staphylococcal, gram-negative bacillary sepsis, tuberculosis, salmonellosis), amoebiasis, giardiasis Hepato-toxines: alcohol, Amanita, acethaminophen; Drugs: isoniazide, macrolides, sulfonamides, metildopa, valproic acid, paracetamol, non steroidal anti-inflammatory drugs, halothane and derivatives, antidepressants, anti-HIV, ketoconazole Chemical agents: carbon tetrachloride, tetrachloroethylene Metabolic disorders: Wilson’s disease, Reye’s syndrome During pregnancy: acute liver steatosis 2.Chronic hepatocellular diseases Chronic active hepatitis Cirrhosis Neoplasia Neonatal hepatitis Autoimmune hepatitis Alfa1-antitripsin deficiency III. Biliary obstruction Extrahepatic Gallstones Neoplasia Sclerosing colangitis Biliary stricture Pancreatitis Parasites Intrahepatic Cholestatic viral hepatitis Postoperative cholestasis Benign recurrent cholestasis Primary biliary cirrhosis Chronic inflammatory bowel disease Biliary atresia Treatment The aim of the treatment is to maintain comfort and nutritional balance. Bed rest is indicated. A high-calorie, high-protein diet has been shown to reduce the length of time in hospital. The adequate calories must be provide: 30-35 kcal/kg, mostly from carbohydrates. Low-fat diet is frequently advised. Avoidance of alcohol is recommended for some months after acute infection. Patients with severe anorexia, nausea or vomiting occasionally need nutritional support. Meat can be introduced when urine exam for bilirubin and urobilinogen are back to normal. In cholestatic hepatitis hypertonic glucose is used. Prevention Now, there is available a double vaccine against A and B hepatitis viruses (Twinrix) HEPATITIS B The hepatitis B virus (HBV), discovered in 1966, infects more than 400 million people world-wide. Epidemiology Areas with high prevalence Areas with low level of endemicity The distribution of hepatitis B infection varies greatly throughout the world. Modes of trasmission Contact transmission through body secretions: semen, vaginal secretions, blood, saliva Maternal-neonatal transmission: 5 –10 % of neonatal HBV infections result from in utero infection Percutaneous transmission: drug abusers, health care workers Blood transfusions. Virologic characteristics HBV belongs to Hepadnaviruses (DNA-woodchuck hepatitis virus, the duck hepatitis virus and other avian and mammalian variants). The hole VIRION – the Dane particle is a 42 nm sphere, which contains: the core enclosing the DNA and an envelope material , which is found abundantly in the circulation, shaped as small spheres and rods with an average width of 22 nm. The viral genome is represented by a double – stranded circular DNA of approximately 3200 pairs of bases that encodes 4 overlapping open reading frames: 1. 2. 3. 4. S, for the surface or envelope gene C, for the core gene X, for the X gene P, for the polymerase gene HBV replication cycle The replication cycle of HBV begins with attachment of the virion to the hepatocyte. Inside the hepatocyte nucleus the viral genome is converted into a covalently closed circular DNA (cccDNA). The cccDNA is Serologic makers of importance in HBV infection Hepatitis B core antigen (HBcAg) - represents the nucleocapsid that encloses the viral DNA. Hepatitis B ″e″ antigen( HBeAg). It is a circulating peptide derived from the core gene and then modified and exported off the liver cells and serves as a maker of active viral replication. DNA polymerase (encoded by the P gene) – also serves as a witness of reserve transcriptase activity. Antibodies against HBsAg (anti – HBs) are produced as a response to the presence of the envelope (surface) Ag: Antibodies against HBcAg (HBcAb) are detected virtually in all patients who have ever been exposed to HBV: they are not protective IgM subtype antibodies are associated with acute infection; usually they disappear within 4-8 months after acute infection. Antibodies against HBeAg (HbeAb) appear once the antigen has been cleared Immune response A. Immune response to acute HBV infection Recovery from acute HBV infection is dependent on: - B cell response with production of antibodies to pre-S and S antigens - T cell response. B. Immune response to chronic HBV infection In patients with chronic HBV infection the HBV-specific CTL response is weak and limited to one or a few epitopes. Immune tolerance in perinatally acquired HBV infection Immune tolerance to HBV is manifested by: A high rate of progression to chronic infection Lack of disease activity in the presence of very high level of virus Very low rate of spontaneous or interferon-induced HBeAg seroconversion. Pathogenesis 1. Immune-mediated liver injury HBV-related liver injury is related to: a. CTL-mediated lysis of infected hepatocytes; b. Non-cytolytic pathways via the release of cytokines. 2. Direct cytopatic effects HBV is in general not a cytopatic virus. Direct cytopathic liver injury can occur when the viral load is very high (as in fibrosing cholestatic hepatitis). Hepatocarcinogenesis Life cycle of HBV in the human host 95% of infected neonates become asymptomatic chronic HBV carriers 30% of children of 3months – 6years become asymptomatic chronic HBV carriers only 3 –5% of adults become asymptomatic chronic HBV carriers. The Four Stages of Hepatitis B Infection are: 1. First Stage (Immune Tolerence) is defined as incubation period 2. Second stage (period of symptomatic hepatitis): In patients with acute HBV infection - it lasts 3 – 4 wks; In patients with chronic disease, stage 2 may persist for > 10 years possibly leading to cirrhosis. 3. Third stage (end of active viral replication) HBeAg is no longer present. HBeAb become detectable. There is a marked decrease in viral DNA. ALT becomes normal. The patient remain + for HBsAg. 4. Fourth stage (immune stage) HBsAg is absent; HBV DNA is absent HBsAb is positive testifing the development of full immunity. Some factors can affect the evolution through the four stages, like: the genetic predisposition of the host, the presence of other viruses, the treatment with immunosuppressive agents, sex, infections with HBV mutants. HBV variants HBV mutant that are detected clinically confer survival advantage over the wild type virus by: - evading host immune response - enhancing virus replication. Mutations in the precore, S and P genes have been most extensively studied. Precore mutants S mutants Mutations in the HBV gene have been reported in infants born to carrier mother who developed HBV infection despite vaccination, and in liver transplant recipients who developed HBV reinfection despite prophylaxis with hepatitis B immune globuline (HBIG). Clinical manifestations I. Asymptomatic hepatitis A. Subclinical infection: abnormal blood tests, without jaundice and symptoms; B. Inapparent infection: no symptoms or biochemical abnormalities are present, but serologic tests are positive II. Symptomatic hepatitis A. Anicteric hepatitis – B. Icteric hepatitis Icteric phase: - dark urine, lightening of stool color – a few days before onset of jaundice, - as jaundice progress the constitutional symptoms become less severe, - duration of jaundice is 1 – 2 months after its onset. The physical findings are similar to those described in HAV, adenopathy, small spider angiomata. Extrahepatic manifestations of viral hepatitis a. transient serum-sickness like syndrome: b. polyarteritis nodosa: c. glomerulonephritis d. mixed cryoglobulinemia e. acrodermatitis of childhood (Gianotti’s disease):. Laboratory features Blood chemistry was shown above. Minor elevation of ALT values may persist for a few months, even if the bilirubin level turned normal. Serologic Tests HBsAg is the first identifiable marker (before the level of ALT start to Increase) by ELISA, RIA. It’s persistence beyond 6 months suggests the development of HBV carrier state. HBeAg it will be detectable in the next days to weeks after the appearance of HBsAg and in uncomplicated HBV infections it will disappear priory to the HBsAg. HBeAb become detectable shortly after the disappearance of HBeAg. They may persist for long periods. DNA- HBV: is detectable during the early phase of infection and → undetectable several wks later. HBcAb (anti - HBc) are detectable in serum shortly after the HBsAg′s discovery and before the appearance of anti-HBs. IgM anti – HBc peak in several weeks from the onset of infection; is the most sensitive test for acute HBV infection. They persist for 4 to 8 months after their appearance. IgG anti – HBc are synthetised in late convalescence and are found thereafter for years, but levels decline slowly. Anti – HBs. As the titer of HBsAg declines, corresponding antibodies become detectable and reach a peak level in a few months. They are - neutralizing, 0 1 2 3 4 5 6 7 8 9 10 Evolution of HBV infection 1. 2. 3. 4. 5. 6. Full recovery Fulminant hepatitis Persistence of HBsAg Chronic hepatitis B Cirrhosis Hepatocellular carcinoma 1. Full recovery is assumed if the ALT reaches normal or close to normal levels and if HBsAg becomes negative in EIA. This takes more than 6 months in some cases. 2. Fulminant hepatic failure (FHF) was defined as a potentially reversible condition, the consequence of severe liver injury, in which encephalopathy developed within 8 weeks of the appearance of the first symptoms, in the absence of pre-existing liver disease Pathophisiology Pathophisiologically, the terminal event in this illness is injury and/or death of hepatocytes. Although the actual pathogenesis is not well understood, this damage is probably due to liberation of chemical and immunological mediators. FHF is announced by some signs, such as: severe jaundice (> 15 mg% in adults or > 7 mg% in infants); mild jaundice does not excluded the fulminant hepatitis; persistence of vomiting and anorexia after the jaundice have installed; presence of bleedings persistence of fever in the presence of jaundice quickly decreased in size of liver; tachicardia instead of bradicardia in an icteric and afebrile patient foetor hepaticus. In stage I coma, mild mental changes are present, but asterixis is minimal or absent. Stage II coma is marked by worsening of mental changes and definite asterixis. In stage III coma, the patient develops stupor and semicoma, but can still be roused. With stage IV coma, the patient no longer is arousable, and there may or may not be a response to deep pain stimuli. Patients with hepatic failure, may demonstrate other neurologic sign - flapping of the tongue, involuntary movements, long-tract signs, and decerebrate posturing. Biological data that announced the FHF are: decreased of Prothrombin - time (Quick index < 30%) decreased of plasminogen (<20%) raised blood ammonia (>120 mg%) leukocytosis with increased level of neutrophils sudden decreased of ALT. Table 2. Complications of FHF and their treatment: Cerebral oedema *Monitor intracranial pressure (extradural monitor) if encephalopathy reaches grade III or IV *Avoid manoeuvres that increase intracranial pressure *Control restlessness *If intracranial pressure > 20-30 mm Hg, apply: 1.Hyperventilation (PaCO2 25 -3- mm Hg) then 2.Mannitol 0,5 g/kg in bolus then 3.Haemodialysis or haemofiltration then 4.Barbiturate coma Renal failure Bacterial infection Circulatory dysfunction Haemorrhagic complications Hypoglicemia Other complications *Avoid arterial hypotension, nephrotoxic drugs, control infection *Dopamine 2 - 4 microgm/min *Haemodialysis or haemofiltration *Careful hygienic measures *High index of suspicion of infection *Daily cultures of blood, urine, and other biological fluids *Start empiric antibiotic therapy *Possible selective intestinal decontamination *Possible parenteral antibiotic prophylaxis *Insert pulmonary-artery catheter and measure indicators of tissue hypoxia frequently *Avoid vasoconstrictors *Possibly treat with N-acetylcysteine *Upper gastrointestinal bleeding; ranitidine or sucralfate *Fresh frozen plasma only in cases of bleeding and before invazive procedures *Measure blood glucose concentration every 1 hour *Intravenopus hypertonic glucose *Pulmonary oedema, respiratory alkalosis, hyponatremia, hypophosphatemia, pancreatitis, aplastic anaemia Persistence of HBsAg for more than 6 months after onset in high concentrations (>100 PEI units/ml) means that: (1) the acute hepatitis B evolved to chronicity; (2) a pre-existing chronic HBV infection was superimposed by an acute hepatitis of another etiology; or (3) the acute disease may actualy be an exacerbation of chronic hepatitis B. Full recovery is assumed if the ALT reaches normal or close to normal levels and if HBsAg becomes negative in EIA. Chronic hepatitis B Clinical manifestations of chronic hepatitis are mild and non-specific : lack of energy, malaise, ease fatigability, myalgias, arthralgias, and skin rash. The evolution is usually intermittent with acute exacerbations consisting on: abdominal discomfort, nausea, weight loss, dark urine, jaundice, loss of appetite. Cirrhosis The above mentioned symptoms are more important, associated or not with signs of hepatic encephalopathy, blood coagulation is decreased, serum albumin is decreased; the ALT levels are only moderately enhanced, and often fluctuate. The liver status can be assessed by histology, liver function tests, and determination of serum proteins with liver metabolism. Hepatocellular carcinoma In highly epidemic areas, persons positive for HbsAg are 100 times more likely to develop HCC compared to those who have anti-HBs. Abdominal imaging by ultrasound of HbsAg-positive patients with liver cirrhosis is advisable at intervals of 3-4 months. Complications Neurological complication: encephalitis, meningoencephalitis, GuillainBarre syndrome, mononeuritis affecting cranial or peripheral nerves. Myocardial involvement: myocarditis and electrocardiographic changes Pancreatitis Pleural effusions and ascites Aplastic anemia Polyarteritis nodosa Membranoprolipherative glomerulonephritis Leucocytoclastic vasculitis Treatment The main aims of treatment of chronic HBV infection are: Viral suppression (as shown by loss of HBeAg with or without seroconversion to anti-HBe) and a decrease in HBV DNA; Reduction in liver damage (return to normal values of serum aminotransferases, histological improvement on liver biopsy samples; Complete eradication of the virus (as shown by loss of HBsAg, with seroconversion to anti-HBs) and the absence of HBV DNA detection. Agents for the treatment of chronic hepatitis B Interferon alfa – it is given parenterally either 5 MU daily or 10 MU three times per week with loss of HBeAg and HBV DNA in only 20% of cases, and loss of HBsAg in only 6% of cases. Lamivudine Entecavir Telbivudina Adefovir Tenofovir Hepatitis B vaccines Long-term immunity against HBV infection is conferred by the presence in the blood of adequate levels of anti-BHs, the antibody to the surface antigen. It is generaly accepted that the threshold anti-HBs titre for protection is 10mIU/ml. It is significant to note that a 3-dose schedule of Engerix-B (10microg/dose) alone gives protective efficacy rates of 94,8%- not significantly different from the efficacy rates of 97,6% and 97,4% for vaccinees who also received HBIG. Passive immunization A special immunoglobulin is produced from donors who are naturally immune and boosted with plasma-derived vaccine. Passive immunization is recommended together with active immunization to prevent HBV infection in newborns from HBsAg and HBeAg mothers, and after proven accidental exposure of a non-immune person. HEPATITIS D Hepatitis D virus is a defective RNA virus that uses HBsAg to produce delta hepatitis. Transmission is similar to that of HBV. A high prevalence of HDV infection is seen in South America, Central Africa, southern Italy and Eastern European countries. Acute HDV infection occurs in two forms depending on the state of HBV infection: 1) Co-infection-acute delta hepatitis occurs simultaneously with acute hepatitis B. Most patients in this situation recover. The rate of fulminant infection is higher than in superinfection. 2) Superinfection- acute delta hepatitis developed on a chronic hepatitis B. Laboratory data Delta antigen can sometimes be detected in serum during the early phase of acute delta hepatitis. HDV RNA can be detected in the serum and liver using molecular hybridization technology. Anti-HDV arises with the disappearance of the Delta antigen. HBsAg is present concomitantly with IgM anti-HBc in early phases of coinfection and with IgG anti HBc in superinfection. Diagnosis of chronic HDV infection involves: High titers of anti HDV (>1:100 by radioimmunoassay) Detection of HDV antigen Persistence of IgM anti HDV in serum. Evolution Chronic delta hepatitis is more severely than chronic hepatitis B alone or chronic hepatitis C, and 60-70% of patients develops cirrhosis. 0 2 4 6 8 10 12 24 32 0 2 4 6 8 10 12 24 32 0 2 4 6 8 10 12 24 32 HEPATITIS C It is a small single-stranded RNA virus that is distantly related to members of the Flaviviridae family (e.g., Dengue viruses, Yellow fever virus, and the Japanese encephalitis virus). According to WHO data, it is estimated that 3% of the world population has been infected with hepatitis C virus. Therefore, there may be more than 170 million chronic carriers in the world Modes of transmission 1. Blood products 2. Intravenous drug users 3. Donor organs (4. The importance of sexual transmission is controversial. The frequency of HCV transmission to female sexual partners was 5 times higher when HIV was also transmitted, possibly because co-infection with HIV leads to higher levels of HCV in serum. 5. The risk of developing hepatitis following needle-stick exposure is 6. Vertical transmission of this virus –the risk of transmission is much lower than for hepatitis B infection. Nosocomial risk is a major point of concern, as documented in oncology units. Pathogenesis Hepatitis C virus is a member of the Flaviviridae family, which includes the flaviviruses and pestiviruses. that encodes a large single polyprotein of 3000 amino-acids: the N-terminal one third harbors the structural proteins; the C-terminal two thirds contains the nonstructural proteins: C22-3 Antiviral immune response Th1: interferon-gamma and interleukin-2, which are important stimuli for the development of the host antiviral immune responses, including cytotoxic T-lymphocyte (CTL) generation and NK-cell activation Th2: interleukin-4, interleukin-5 and interleukin-10, which enhance antibody production and downregulate the Th1 response. The imbalance between the Th1 and the Th2 responses is implicated in disease progression and the inability to clear the infection. Humoral immune response Cellular immune response The cellular immune response has the ability to recognize and eliminate Clinical manifestations HCV infection is infrequently diagnosed during the acute phase of infection. Incubation period is usually 7 to 8 weeks (range 2 to 26). The majority of persons have either no symptoms or only mild symptoms. Symptoms of acute hepatitis usually consist of: jaundice, malaise and nausea. A characteristic feature of all these patients is marked fluctuation of aminotransferase level. Although the aminotransferase level (ALT) may occasionally peak as high as 1000 to 2000IU/L, Extrahepatic manifestations of HCV infection are associated with autoimmune or lymphoproliferative states Lymphoproliferative disorders 1. Mixed cryoglobulinemia.. 2. Lymphoma. Other extrahepatic diseases Autoimmune thyroiditis Dermatological manifestations- apart from the palpable purpura due to leukocytoclasic vasculitis (the most frequently seen dermatological manifestation of HCV-related EMC) other cutaneous disorders are: sporadic porphyria cutanea tarda and cutaneous/mucosal lichen planus Sjogren’s syndrome Idiopatic pulmonary fibrosis Mooren corneal ulcers. Tests for HCV There are still no tests for antigens of hepatitis C virus in serum. Infection is usually diagnosed by detecting hepatitis C virus antibodies based on immunoassay detection. 1. HCV antibody testing 2. Detection of serum HCV RNA Detection of serum HCV RNA by PCR is the best test for early diagnosis of acute HCV infection (it is positive as soon as 1 week after exposure). 3. HCV genotypes. HCV was classified into 6 types (1 to 6) each including subtypes (a, b, c...) 4. Quasispecies There is a correlation between a high quasispecies heterogeneity an a more severe liver disease and a poor response to interferon therapy. 0 1 2 3 4 5 6 12 24 36 48 60 120 Evolution The infection becomes chronic in most cases (80%), and chronic infection is typically characterized by a prolonged period in which there are no symptoms. . At the cirrhotic stage, about 3-5% of patients per year develops hepatocarcinoma, which may develop in up to 50% within 10 years. Chronically infected individuals generally have fluctuating or continuously abnormal levels of ALT and are viraemic. Treatment of Hepatitis C infection - Prior to treatment, the investigation should: ascertain that chronic hepatitis C virus infection (HCV) is present; exclude contraindication of treatment; evaluate histological findings determine the viral genotype, and for genotype 1 patients, also, the viral load. Exclude contraindication for treatment Contraindications for interferon (IFN) and IFN/ribavirin combination therapy are: - Pregnancy or no optimal contraception(ribavirin) - Severe heart disease - CNS dysfunction/epilepsy - Decompensated liver disease(interferon) - Organ transplant, except liver transplant - Severe renal failure(ribavirin) - Untreated severe anemia(ribavirin) - Hemoglobinopathies(ribavirin) - Autoimmune hepatitis(interferon) - Ongoing or recent drug or alcohol abuse - Leukopenia(WBC<1500/mm3, neutrophols<750/mm3) - Thrombocytopenia(<50000/mm3) Relative contraindications: -Psychiatric disease/depression -Not well controlled thyroid disease or diabetes melitus -Autoimmune disease -Mild bone marrow suppression. Evaluation of the histological damage Patients with mild histological inflammation without fibrosis will probably not develop serious liver disease, not even with prolonged followup. A second histological evaluation after 2-5 years is therefore recommended for this patient cathegory. Cirrhotic patients, with manifest disease, should only be treated if they are in compensated phase, while those with decompensated cirrhosis should be evaluated and considered for possible liver transplantation. Recommend treatment if: - HCV-RNA is detectable - ALT above the upper normal reference level - No contraindications for treatment - Liver biopsy showing fibrosis stage >=2 and ongoing inflammation or/ fibrosis stage 1 and inflammation grade >2 Treatment with: -alfa-IFN 3MU s.c., 3 times a week + ribavirin 1000-1200 mg /day. Non-responders to previous IFN monotherapy: -Lack of sufficient information - no recommendation given. Table 1. Treatment recommendations Patients Previously untreated patients -Genotype other than 1 or genotype 1 and viral load < 2-3 million genome copies/ml -Genotype 1 and viral load > 2-3 million genome copies/ml Previous relapse after IFN monotherapy -All genotypes -If contraindication for ribavirin Treatment length -Treatment for 24 weeks -Treatment for 48 weeks. -24 weeks treatment -IFN monotherapy weeks for 48 Pegylated Interferon Indication of pegylated interferon (with ribavirin): - first intention therapy of patients with: fibrosis-stage 3 (Knodell or Metavir score), necroinflamatory score>9, hemophilia/thalasemia, age<55 years - recurrences /absence of response after the standard therapy with interferon and ribavirin, coinfections. Inhibition of viral replication might also be achieved by: Antisense oligonucleotides that bind to specific sequences in the HCV RNA. Ribozymes that catalyze cleavage of HCV RNA. Gene transfer of interfering proteins, that specifically interrupt virion assembly in the hepatocyte. HEPATITIS E Hepatitis E virus was identified by Balayan et al. (1983) in human fecal samples. In 1995 this virus has been classified within the Caliciviridae. HEV is a non-enveloped, RNA virus with single-stranded genome Epidemiology Hepatitis E could be a sporadic disease or may evolve in outbreaks with contaminated drinking water as the source of infection. The most frequently involved regions are: India, China, Pakistan, Egypt, Algeria, Sudan, Ethiopia, Mexico. HEV infections generally occurred after the age of 16 years Pathogenesis Pathological changes in the liver include hepatocyte necrosis and inflammatory cell infiltration Clinical manifestations Incubation period has an average of 40 days. HEV produces an acute, self-limited disease without progression through chronic hepatitis. The prodromal symptoms include: malaise, anorexia, abdominal discomfort. In women infected during the third trimester of pregnancy was noted a high rate of fulminant disease and a 15-20% case-fatality rate. Diagnosis 1. Detection of HEV-RNA by PCR 2. Detection of HEV-Ag by electronic immunomicroscopy 3. Detection of HEV-antibody: - immunofluorescence inhibition - ELISA (IgM, IgG) - Immunotransfert (IgM, IgG) GB VIRUS-C/HEPATITIS G VIRUS Virology and tissue tropism GBV-C/HGV is an enveloped RNA virus which belongs to the Flaviviridae family. The envelope 2 (E2) region encodes a glycoprotein which is located on the outer surface of the virion. At least 4 major genotypes exists. GBV-C/HGV may replicate in human peripheral blood mononuclear cells and endotelial cells, in liver tissue, spleen and bone marrow. Prevalence and transmission There is a high prevalence of viremia in healthy persons in Europe and North America (1-4%) and a higher prevalence rate in South America and Africa (10-33%). GBV-C/HGV may be transmitted via blood/blood products, by sexual contacts, by close social contact, and from mother to infant. Clinical features The majority of adults have a transient infection with clearence of viremia and development of E2 antibodies. GBV-C/HGV viremia after vertical or perinatal transmission may persist for at least 36 months. Diagnostic 1. Reverse transcription polymerase chain reaction (RT-PCR) – 2. PCR is used to diagnose ongoing infections and does not give any information on a resolved infection (may underestimate the prevalence/occurrence of these infections). 3. EIA 4. In situ hybridization to detect GBV-C RNA 5. Immunofluorescence. 6. Liver biopsy Infection with TT Virus TTV is a unenveloped, single stranded, circular DNA virus that was isolated from a Japanese patient with post-transfusion hepatitis of unknown etiology. Although TTV DNA titers have been shown to be closely correlated with aminotransferase levels in the sera of some patients during post-transfusion hepatitis, no clear association between TTV infection and human liver disease has been establish at this time. TTV is a parenterally transmissible virus TTV DNA was detected in 10% of normal population in England, in 34% of voluntary US blood-donors and in 47% of patients with acute and chronic non A-G hepatitis. Very high prevalences of viremia (>50%), observed in healthy populations in different countries, indicate that persistent infection is common and that routes of transmission other than the parenteral route do exist. However, the main mode of TTV transmission remains to be determined. RICKETTSIAL DISEASES Egidia Miftode The family Rickettsiaceae comprises a group of small, gram-negative, obligate intracellular bacteria that normally infect animals and sometimes cause disease in humans. Human rickettsial diseases are zoonoses. The definitive hosts for most rickettsia are rodents or other mammals, whereas vectors are ticks, mites, fleas or lice. The rickettsial diseases that affect humans fall into four divisions: 1. the spotted fever group 2. the typhus fever group 3. the scrub typhus group 4. a group of conditions including: - Q fever - ehrlichiosis and - acute febrile cerebro-vasculitis (with presumed but unproven rickettsial etiology). - Infections caused by Bartonella (formerly Rochalimaeae), organisms which were formerly grouped with the rickettsiae, are described separately. Pathogenesis Rickettsia are introduced into the human body in various ways: Directly by the bite of vectors (ticks or mites); Contamination of the skin by infective faeces from the vectors (fleas or lice); Contamination of the oropharyngeal mucous membranes by inhalation of aerosolized faeces from fleas or lice Table 1. The major rickettsial diseases Disease Organism Vector Host Geographic distribution Ticks Rodents Ticks Rodents -North Asian tick R. sibirica typhus -Queensland tick R. australis typhus -Rickettsialpox R. akari Ticks Rodents N, C., S America Africa, Asia, Medit.basin Asia Ticks Rodents, marsupials Mouse -Oriental spotted R. japonica fever Typhus group -Epidemic typhus R prowazeki unknown unknown Spotted fever group -Rocky Mountain Rickettsia spotted fever rickettsii -Boutonneuse fever R. conorii -Murine typhus Scrub typhus group -Scrub typhus Other genra -Q fever Mite Body louse R. typhy Rat flea R. tsutsuga- Larvae mushi of mites Coxiella burnetii -Human monocytic Ehrlichia Ehrlichiosis chafensis -Human granulo- Ehrlichia sp cytic Ehrlichiosis None Humans N Australia Possibly wordwide Japan Wordwide rodents wordwide Rodents/bir Asia, Pacific ds island, Austr. Cattle, Worldwide goats,sheep Unknown Possibly worldwide Amblyo mma american um ticks unknown ROCKY MOUNTAIN SPOTTED FEVER Possibly worldwide RMSF is caused by Rickettsia rickettsii the vector for this pathogen being the wood tick or the common dog tick. The disease is found throughout the Western hemisphere. Clinical features Laboratory findings Blood: normal or low total white blood cell count with a market left shift, thrombocytopenia, hyponatremia, elevated liver enzyme concentrations, and increased level of bilirubin; CSF can be normal or there is a mild CSF pleocytosis (<200/mmc), CSF protein concentration is elevated; Electroencephalography is usually abnormal, but nonfocal; Serologic confirmation: immunofluorescence, PCR, ELISA. Treatment - Tetracycline in children over 8 years of age (20-50 mg/kg/day) or in adults (500 mg four times daily) - Chloramphenicol (50 mg /kg/day in four doses) for children under 8 years of age - Ciprofloxacin OTHER SPOTTED FEVER GROUP RICKETTSIOSES These infections include: boutonneuse fever (Mediterranean spotted fever), East African tick typhus and South African tick-bite fever, all caused by R. conorii; North Asian tick typhys (by R sibirica), Queensland tick typhus (R. australis) and Oriental spotted fever (R. japonica). Infections caused by R. conorii Laboratory findings - normal/low white blood count (with granulocytosis in elderly and lymphocytosis in young) - thrombocytopenia - abnormal liver function tests - CSF: lymphocytic pleocytosis, hyperglycorrachia - Histology: cerebral/cerebellar perivascular mononuclear infiltrates - Identification of ricketsia by immunofluorescence, PCR Treatment: EPIDEMIC TYPHUS Laboratory findings - leucocytosis with neutrophilia - abnormal level ofliver enzymes - CSF pleocytosis Clinical manifestations of epidemic typhus and the pathology of typhus in the CNS resemble that of RMSF. Treatment: tetracycline or chloramphenicol need to be continued for 2 to 3 days after defervescence. If a recurrent illness occurs after early treatment a second course of therapy are needed. Mortality rate ranges from 15 - 20% to as high as 60% in debilitated patients in severe epidemics. BRILL-ZINSSER DISEASE This is a late recurrence of epidemic typhus associated with a wanning immunity to a past infection to R. prowazekii. Clinical manifestations are less prominent that in the primary disease: onset with fever, headache, and rash; milder neurological complaints. Differentiation of Brill-Zinsser disease from primary epidemic typhus can be made by: antibody analysis (IgM antibodies are present in primary disease and IgG in Brill-Zinsser disease), CSF analysis (usually normal in Brill-Zinsser disease). The treatment is the same as in primary typhus. Q FEVER Coxiella burnetti is the etiologic agent of Q fever. It is most often a non specific, self limited febrile illness, but it may present as: atypical pneumonia, endocarditis, granulomatous hepatitis, osteomyelitis or a variety of neurological syndromes. Human acquires C.burnetti from infected animals or animal products, probably by direct inhalation. Clinical features Incubation is about 20 days. The patient develops fever, chills, myalgias, headache, fatigue. Laboratory features There is a normal WBC count. Liver function tests are normal. CSF is normal. CT and MRI scans of the head in patients with Q fever meningoencephalitis have been normal. Specific immunofluorescence in tissues or serology can establish this diagnosis. In chronic Q fever, granulomatous inflammation occurs. Focal accumulations of mononuclear cells, giant cells and neutrophils with a central clearing have been described on the liver and bone marrow. Treatment Acute Q fever is typically a self-limited illness, but, for Q fever with pneumonia, hepatitis or CNS disease are recommended: tetracycline or chloramphenicol for 2 weeks erythromicin, trimetoprim-sulfametoxazole, and rifampin are also effective. EHRLICHIOSIS The genus Ehrlichia includes species of intraleukocytic rikettsiae which are primarily animal pathogens; some also cause human diseases. There are two leading ehrlichial diseases: 1. Human monocytic ehrlichiosis (HME) is caused by E. chaffensis, which infects mononuclear leukocytis and causes a febrile illness resembling RMSF: fever, chills, myalgias, headache and a history of a tick bite. 2. Human granulocytic ehrlichiosis (HGE) is caused by an as yet unnamed species that causes high fever, chills, headache, myalgias. Laboratory features Treatment: atients with HME or HGE usually respond well to tetracycline or doxycycline given in the same doses used for RMSF. Human disease caused by Bartonella species I. Bacillary angiomatosis (BA) and bacillary peliosis hepatis A. Bacillary angiomatosis (BA) Cutaneous lesions of BA are the most frequently identified form of BA, but these vascular proliferative lesions also occur in other organs including: bone, brain, lymph nodes, gastrointestinal and respiratory tracts. B. Bacillary peliosis hepatis (BP) Treatment The drugs of first choice in the treatment of BA or BP are: 1. erythromycin or 2. doxycycline; 3. rifampin may have clinical efficacy as adjunctive therapy in patients who have CNS disease. II. CAT scratch diseases (CSD) CSD occurs almost exclusively in immunocompetent individuals. Before B. henselae was documented as the causative agent, diagnosis of CSD was made when three of four clinical criteria were met: 1) a history of cat contact and the presence of a primary inoculation site, 2) development of lymphadenopathy approximately 2 weeks after the primary inoculations, 3) a positive CSD skin test result and 4) histopathological findings characteristic of CSD in biopsied lymph node tissue. Treatment Treatment of immunocompetent patients with uncomplicated CSD is Azithromycin 500 mg p.o. x 1, then 250 mg/d x 4 (children 10 mg/kgc). In immunocompromised patients, the treatment is Clarithromycin/Azithromycin, Ciprofloxacin, Erythromycin or Doxycyclin. Relapsing Bacteremia and Endocarditis Bartonella bacteremia and endocarditis have been reported to occur in both immunocompromised and immunocompetent hosts in the absence or presence of focal Bartonella infection. TETANUS Egidia Miftode Tetanus is a disease of the nervous system characterized by persistent tonic spasm, with violent brief exacerbations. The onset is acute and the spasm almost always commences in the muscles of the neck and jaw, causing trismus, and involves the muscles of the trunk more than those of the limbs. Microbiology and Pathophysiology Clostridium tetani is an obligate anaerobic rod Two exotoxins, tetanospasmin and tetanolysin, are released by some strains of the organism while it is actively dividing. Pathophysiology Tetanospasmin act at three levels: central motor control, autonomic function and neuromuscular junction. The 150-kD peptide tetanospasmin inhibits release of inhibitory neurotransmitters g-aminobutyric acid (GABA) and glycine. The resulting imbalance in the central nervous system predisposes the patient to reflex spasms. The process involves three steps: 1. binding to the presynaptic membrane, 2. translation of the toxin to the active site 3. induction of paralysis Clinical manifestations Tetanus can occur in patients with gangrene, burns, decubitus ulcers, septic abortions, intramuscular injections, dental infections, penetrating eye injuries, and umbilical stump infections. The incubation period range from 3 to 30 days (11 days). The disease has four forms: Generalized tetanus. a. The most commonly presenting sign is trismus b. risus c. the typical generalized spasm Other positions are orthotonus and pleurosthotonus d. Generalized spasms can involve abdominal, diaphragmatic, and laryngeal muscles, e. Autonomic dysfunction is the leading cause of death in tetanus patients. Localized tetanus Cephalic tetanus: -Facial paresis is usually present -Ophtalmoplegic tetanus when there is a paresis of the III rd, IV th and VI th cranial nerves. Neonatal tetanus Rating scales for tetanus severity Table 1. Veronesi scale Veronesi scale Incubation period<7 days Period of onset*<48 hours High-risk portal of entry (surgical, abortion,etc) Generalized tetanus Core temperature above 40 C Tachycardia (heart rate >120 in adults, >150 in neonates) Score one point for each: *period from the first symptom to the first reflex spasm. Table 2. Severity and prognosis Score* Severity Mortality 0-1 Mild <10% 2-3 Moderate 10-20% 4 Severe 20-40% 5-6 Very severe >50% *Cephalic tetanus are always scored as sever or very severe Neonatal tetanus are always scored as very severe. Table 3. Modified Ablett scale of severity Group Mild Moderate Symptoms Mild rigidity and spasms More intense spasms and rigidity, accompanied by disphagia Severe Marked rigidity, frequent generalized spasms, disphagia, respiratory compromise/apnea Diagnosis The average annual incidence of tetanus in the world is about 1 million cases. Due to its rarity, the initial clinical symptoms may be missed and the diagnosis may not be made until a generalized tetanic spasm occurs. Diagnosis is established in the presence of a portal of entry, trismus, tonic contraction, sudden spasms and a descending manner of contraction progression. Differential diagnosis Trismus in tetanus must be distinguished by: alveolar ridge abscess, temporomandibular joint arthritis, tonsillitis; Spasms and rigidity must be distinguished by: generalized convulsive status epilepticus, strychnine intoxication, tetany precipitated by hypocalcemia, meningitis, encephalitis, brain hemorrhage; Local tetanus-with transverse myelitis. Complications 1. 2. 3. 4. 5. Respiratory dysfunction: Cardiovascular complications: cardiomiopathy Urinary tract infections and acute renal failure caused by rhabdomyolysis Vertebral compression, fractures, hyperostoses CNS and systemic consequences of hypoxia. Management Management of tetanus involves: A. Neutralizing wound and circulating toxin, B. Eradicating the source of toxin C. Standard supportive care D. Treating related complications E. Treatment of the portal of entry Mortality Even with today's advanced level of care, during 1995-1997 the case fatality ratio of tetanus was 11%. LEPTOSPIROSIS Egidia Miftode Leptospirosis is a zoonotic disease that affects human on all continents, in both urban and rural areas. Clinical presentation of this febrile illness may include in severe forms liver involvement, renal failure, meningitis, myocarditis, hemorrhagic pneumonitis, and hemodynamic collapse. The first description of the leptospirosis was done in 1886 by Weil. Etiology Etiologic agent is a spirochete of the genus Leptospira. Leptospires are aerobic bacteria that appear as flexible, thin and motile, helicoidal rod (6-20 microm in lenght and 0,1-0,2 microm in diameter). Epidemiology The reservoir of Leptospira is represented by wild, domestic or peridomestic animals and the spirochetes are excreted in urine, placenta or amniotic fluid At risk to acquire Leptospira are occupational activities such as abattoir workers, veterinarians, rice farmers, livestock workers, or recreational activities (swimming in lakes etc). Table 1. Serovars of Leptospira spp and the potential animal reservoir Serovars L. grippotyphosa and pomona L. canicola, icterohemorrhagiae, pomona L. australis, bataviae, hardjo, gryppotyphosa, icterohemorrhagiae and pomona L. pomona Potential animal reservoir cattle and sheep Pig Rodents Horse Pathogenesis Leptospira enter the body through skin lesions, water logged skin, mucouse membrane or conjunctivae, aerosol inhalation. Septicemic phase. Leptospira are cleared from the circulation by the reticuloendothelial system. Circulating antibodies are involved in the development of aseptic meningitis, uveitis, iritis, iridocyclitis chorioretinitis. Other mechanisms of leptospiral pathogenesis have been suggested to Clinical manifestations The disease may evolve as a subclinical illness, self-limited disease, or as a severe disease. Incubation period is 7-14 days (range 5-30 days). The evolution of leptospirosis is biphasic: 1. The acute septicemic phase: - the onset is abrupt with high fever, chills, headache, myalgia, conjunctival suffusion, back pain, abdominal pain, nausea, vomiting, cutaneous eruption (maculopapular/macular/urticarial rash), cough; - physical examination reveals hepatomegaly, splenomegaly, muscle tenderness; 2. Immune phase: - lasts from 4 to 30 days - recurrence of fever - meningeal symptoms and signs - rash, uveitis. In severe form of the disease (Weil’s syndrome) symptoms and signs of the initial phase progress to a life-threatening illness liver involvement); renal failure; circulatory collapse,; severe hemorrhagic pneumonitis The convalescent period may have duration of several months. Laboratory features Nonspecific features - Leukocytosis with neutrophilia, the sedimentation rate is elevated - Thrombocytopenia - Proteinuria, pyuria, microscopic hematuria, increased blood urea nitrogen - Increased bilirubinemia and liver enzymes, hypoprothrombinemia - CSF examination during the immune phase reveals pleocytosis (<500/mmc), and elevated proteinorrachia; - Chest x-ray abnormalities (opacities, pleural effusions) - Electrocardiographic abnormalities even without evidence of heart disease (inverted T waves, tachycardia, small QRS complexes). Specific diagnosis 1. Isolation of leptospires 2. Direct detection using: 3. Seroconversion) 4. IgM ELISA assay). 5. The indirect hemagglutination test. Differential diagnosis A febrile illness associated with myalgia and headache (with or without liver/renal involvement) must be differentiated from different entities such as: meningitis, encephalitis, nephritis, influenza, hepatitis, typhoid fever, septicemia, brucellosis, relapsing fever, malaria, toxic shock syndrome etc. Treatment 1. Supportive management: measures to manage the fever, pain, renal failure, liver injury, fluid and electrolyte imbalances, hypotension; 2. Antibiotics: - Penicillin i.v. for severe and late disease - Ampicillin, amoxicillin, tetracycline orally-for mild to moderately severe disease. Prevention includes: - maintaining hygiene, - protective clothing - immunization of animals with leptospira vaccine. ANTHRAX Egidia Miftode, Luminita Sabadis The disease anthrax is produced by the sporulating bacterium Bacillus anthracis, found in the soil in many parts of the world. The spores from infected animal carcasses can contaminate the pasture for many decades and lead to sporadic outbreaks. Epidemiology The disease is naturally one of animals, particularly herbivores, who ingest spores on the grass and from the environment. Until the advent of an effective vaccine, the disease was common in cattle, sheep, goats, horses and pigs, but is now far rarer. Etiology C. anthracis is a large (1x3mm), Gram-positive, aerobic, rod-shaped, nonmotile, sporulating bacillus. The spores are central and thermostable. Like many other members of the genus Bacillus, the spores are remarkably resistant and long-lived in the environment. Pathogenesis Naturally acquired anthrax in humans is generally due to contact with infected animals or their carcasses. Although fatal human disease may occur, B. anthracis is not highly virulent, and in spite of its previous prevalence in the environment and in animals, human infections were not common and are now rare. Three toxins are produced by B.anthracis all thermolabile proteins; Clinical manifestations Cutaneous form is the commonest form of the disease, characteristic black, painless eschar with surrounding oedema. In most cases cutaneous anthrax is self-limited, the lesion resolving within 10 days. In some cases systemic anthrax may develop (malignant edema Inhalation anthrax. Gastro-intestinal anthrax. Meningitis Laboratory findings Gram stain examination of vesicle fluid, CSF, blood Culture of vesicle fluid, CSF, blood Immunofluorescence in those with negative blood cultures, PCR, immunohistochemical staining or a four-fold rise in IgG can confirm anthrax. Electrophoretic immuno-transblot test Termoprecipitation reaction (Ascoli-Cornelson-Toma) (formation of an opalescent ring at the interface between the mixture containing crust and anti-anthrax serum) abnormal radiographs/mediastinal widening in inhalation anthrax total WBC normal or only slightly elevated increased % of neutrophils or band forms Differential diagnosis - Cutaneous anthrax: Staphylococcal lesions, cutaneous leishmaniasis, herpes zoster, herpes simplex lesion, erysipelas, inflammatory edema of the face; - Gastrointestinal anthrax: food poisoning, acute abdomen, shigellosis, yersiniosis - Inhalation anthrax: other pneumopathies - Anthrax meningitis: Listeria meningitis, tuberculous meningitis, meningeal hemorrhage. Treatment Most strains of B. anthracis are susceptible to a wide range of antibacterial agents. The organism was traditionally highly susceptible to penicillins and, until recently, these were the drug of choise. - the first choice is now generally a fluoroquinolone, (ciprofloxacin), although other FQ are probably also effective. Therapy of current inhalation cases: combination intravenous treatment with Ciprofloxacin and Rifampicin plus Clindamycin/ Vancomycin/ Penicillin. Current dosages recommended: Penicillin G (procaine) – 600.000 U im. every 6 or 8 hours Penicillin V – 500 mg orally 3 times a day Doxycycline – 100 mg orally 3 times a day Ciprofloxacin – 500 mg twice a day Prevention Vaccination is not a viable option currently for large numbers of people and supplies are not plentiful. BRUCELLOSIS Dr. Egidia Miftode, Dr. Daniela Leca Brucellosis is an infectious disease characterized by an acute febrile stage with few or now localizing signs and by a chronic stage with relapses of fever, weakness, sweats and vague aches and pains. The pathogen Brucella are smal, gram-negative coccobacilli that are nonmotile and do not form spores. The major cell wall antigen of the brucellae is endotoxic lipopolysaccharide (LPS). Epidemiology Brucellosis is a zoonosis and virtually derives directly or indirectly from animal exposure. Pathogenesis Brucella spp. usually gain entry to the body through abrasions in the skin in the course handling infected animals or their carcasses. Accidental innoculation of the conjunctival sac of the eyes is another route of infection that is especially common among veterinarians using live brucella vaccines. Infection via the respiratory tract is a special risk for abattoir workers engaged in the slaugther of infected animals. Brucella melitensis is generally transmited via ingestion of unpasteurized dairy products.. Bacteria entering lymphatics and hematogenous dissemination is then followed by localization of bacteria in organs rich in elements of the reticuloendothelia system (RES), including liver, spleen, lymph nodes, bone marrow, and kidneys. Host immunity Clinical manifestations The incubation time from exposure to the onset of symptoms is generally 2-3 weeks (range:2-8 weeks) Brucellosis is a systemic infection that can involve many organs and tissues. Symptoms are nonspecific: Fever, sweats, malaise, anorexia, headache, and back pains. An “undulant” fever pattern is observed if patients go untreated for long periods of time. Some patients complain of sweat and a peculiar taste in their mouth. Depression is common, and physical abnormalities may be few. Mild lymphadenopathy occurs in 10-20 percent, and splenomegaly in 20-30 percent of cases. Hepatomegaly is reported in 20-60 percent. Complications Gastrointestinal tract.. Osteoarticular manifestations. Neurologic Respiratory symptoms Genitourinary complications. The hematologic manifestations:. Cutaneous manifestations:. Evolution Brucellosis may evolve as: 1. Acute (typical form), 2. Subacute disease 3. Chronic brucellosis Symptoms in such patients resemble the “chronic fatigue syndrome”. Diagnosis Because the symptoms of brucellosis are nonspecific, it is imperative that the clinician obtain a detailed history, including occupation, exposure to animals, travel to enzootic areas, and ingestion of high-risk foods (e.g. unpasteurized dairy products). Laboratory findings White blood cell (WBC) count is usually normal or depressed. Anemia, leukopenia and thrombocytopenia are common. Diagnosis of brucellosis is made with certainty when brucellae are recovered from blood, bone marrow, or other tissue. The Castaneda biphasic technique, lysis-concentration techniques, polymerase chain reaction (PCR) are rapid diagnostic tests. Serological tests are very useful for diagnosis: - Agglutination assay Rose-Bengal test is easily and rapidly performed (used when there are acute manifestations). - Coombs test and blocking antibodies are recommended in the diagnosis of chronic brucellosis - Immunofluorescence - ELISA - Counterimmunoelectrophoresis Differential diagnosis Brucellosis may be distinguished among many common illnesses that may mimic the most frequent presentations: infectious mononucleosis, septicemia, toxoplasmosis, tuberculosis, hepatitis, systemic lupus erythematosus, thyphoid fever. Treatment Doxycycline 100 mg orally bid (or tetracycline 500 mg orally qid) for 3 to 6 weeks plus streptomycin 1 g IM q 12 to 24 h for 14 days; Doxycycline 100 mg orally bid + Rifampicin 600-900 mg/day; In children < 8 years : thrimethoprim/sulphamethoxazol and either IM streptomycin or oral rifampin for 3 to 5 weeks; Ciprofloxacin 1 g/day and rifampicin 600 mg/day for 30 days Prevention Live-attenuated brucella vaccines were used for human immunization in some countries; however, they were restricted to high-risk personnel in areas of high endemicity. RABIES Egidia Miftode An important histopathologic observation was made by Negri in 1903, when he discovered cytoplasmic inclusion bodies (“Negri bodies”) in the neuronal cells of the brains of rabid dogs. In 1912, Babes published a compendium of cases that defined the natural history and clinical findings of human rabies, which remains one of the most complete documents about this disease. Etiology Rabies virus belongs to the Rhabdoviridae family which includes pathogens that infect a wide variety of mammals, fish, birds and plants. The Rhabdoviridae family consists of two genera: Lyssavirus and Vesiculovirus.. Pathogenesis Most wild mammals can become infected with rabies, but susceptibility varies according with species. Foxes, wolves, coyotes, and jakals are the most susceptible; dogs, bats, raccoons, mangooses and monkeys are intermediate; opossums are quite resistant. There are two routes of rabies virus acquisition: most cases of human rabies occur following animal bites, and nonbite routes of transmission include aerosols, and person-to-person transmission following corneal transplants. Clinical manifestations Incubation period for rabies is very variable – between days and years (usually 30-90 days). Prodrome and early clinical symptoms have a duration of 2-10 days: -paresthesia/pain at site of bite, -malaise, fever, myalgias, headache, -nausea, vomiting, abdominal pain, diarrhea Acute neurologic disease (2-7 days) can be classified in two forms: 1. “Furious rabies”(80%): 2. “Paralytic rabies” (20%): Coma can develop immediately after the onset of disease or it can occur up to 14 days after the onset. Death occurrs an average of 18 days after the onset. Treatment No specific therapy exists for rabies virus infections in humans or animals. Prevention The principles used to control rabies in domestic animals were restriction of movement and mandatory vaccination. Since the early 1980s, orally absorbed vaccines, distributed by hand and by aircraft, have controlled rabies in foxes. A. Pre-exposure prophylaxis A pre-exposure regimen includes the administration of vaccine intramuscularly over 28 days on days 0, 7, 21, and 28. Post-exposure prophylaxis The combination of local wound treatment, passive immunization, and vaccination is uniformly effective. Table 1. Post-exposure prophylaxis Circumstances 1.Person not previously vaccinated The animal was not captured The animal is dead Animal is alive and could be observed 2. Person previously vaccinated Management Immediate cleansing with soap and water Rabies immune globulin (RIG): 20mg/kg of body weight Vaccine: 1 ml of vaccine i.m. in the deltoid area on days 0, 3, 7, 14, and 28. RIG+vaccine Start the prophylaxis and stop in function of laboratory exam (direct fluorescent antibody testing of the animal brain) Start prophylaxis* and stopped if the animal doesn’t present any signs on days 0, 7, 14 Local wound cleasing RIG should not be given Vaccine on days 0 and 3 *Type of exposure: Person licked on a normal skin: no prophylaxis necessary Superficial wounds: vaccination Bites or contamination with saliva of an open wound/mucous membrane: RIG and vaccine. MALARIA Egidia Miftode The four most common species that infect humans are: 1. Plasmodium vivax – may account for 80% of of the infections 2. Plasmodium falciparum - generally confined to the tropics 3. Plasmodium malariae – is sporadically distributed 4. Plasmodium ovale – is confined mainly to central West Africa. The number of imported malaria cases increases. Infected mosquitoes can reach different areas by airplanes. Life cycle and morphology The vector for malaria is the female anopheline mosquito. With the vector blood meal, sporozoites contained in the salivary glands are discharged into the bite wound; Sporozoites are carried to the liver (via the blood), where they penetrate hepatocytes, grow and initiate the preerythrocytic (primary exoerythrocytic) cycle; The sporozoites begin dividing; this schizogony results in large numbers of exoerythrocytic merozoites; After the merozoites leave the liver, they invade the red blood cells (RBC), thus initiating the erythrocytic cycle; A dormant (secondary) schizogony may remain quiscent in the liver resulting hypnozoites; this stage occurs only in P. vivax and P. ovale organisms; Within the RBC, the merozoite (or young trophozoite) is vacuolated, ring shaped, ameboid and uninucleate; malarial pigment is present inside the RBC; Once the nucleus begin to divide, the trophozoite is called a developing schizont; The mature schizont contains merozoites (whose number depend on the species) which will be released into the blood stream; One part of the merozoites are destroyed by the immune system, and others invade RBCs, in which initiate a new cycle of erythrocytic schizogony; Some of the merozoites do not become schizonts, but, develop into the male and female gametocytes; Gametocytes, ingested by mosquito, mature into gametes (in the mosquito gut) The male microgametes penetrate the female microgametes resulting a zygote; Zygote becomes motile and is called ookinete which secretes a thin wall and grows into the oocyst; Within a few days to 2 weeks, the oocyst matures, with the formation of hundreds of sporozoites; some of them reach the salivary glands. Physiopathology Mechanisms of anemia are: Direct RBCs lysis which occurs during the life cycle of the parasite Splenic removal of RBCs Autoimmune lysis of RBCs Decreased RBCs production from bone marrow Increased fragility of RBCs Immunity Some genetic alterations in the RBCs confer natural immunity to malaria: Infants are relatively immune to malarial infection during the first year of life (presence of a large percentage of HbF). Clinical manifestations Incubation period varies between 8 to 40 days, depending on species (table 1). Sometimes may be prolonged for months to years. - Classic paroxysm: cold stage, fever, and sweats; fever may be absent in the early stages of the infection. Paroxysm begins with the cold stage and rigors (lasting 1 to 2 hours) followed by high fever. The last hours body temperature decreased to normal or subnormal. Periodicity of the cycle may not be established during the early stages. - Anemia - Splenomegaly - Other symptoms: lethargy, vomiting, diarrhea, headache. Complications 1. Disseminated intravascular coagulation 2. Cerebral malaria; Table 1. Clinical characteristics of different types of malaria Characteristic P. vivax P. ovale Incubation period (days) Prodromal symptoms -Severity -Initial fever pattern 10-17 10-17 P. malariae P. falciparum 18-40 8-11 ++ Irregular, quotidian + Irregular, quotidian ++ Regular, 72 h Periodicity Initial paroxysm -Severity -Avg duration Duration of untreated primary attack (wk) Duration of untreated inf Anemia CNS involvement Nephrotic syndrome Relapses 48 Moderate to severe 10 3-8+ 48 Mild 10 2-3 72 Moderate to severe Severe 11 16-36 3-24 2-3 5-7 yr 12 mo 20 yr 6-17 mo ++ + + +/- ++ + ++++ ++++ +/- + ++++ + Yes Possible, but usually spontaneous recovery No, but long-term recrudescen ces are recognized No longterm relapses 3. Bilious remittent fever 4. Algid malaria 5. Blackwater fever – Acute renal failure – + Continuous remittent, quotidian 36-48 Laboratory diagnosis Hemoleucograme shows: anemia, leukopenia/occasional leukocytosis, and less frequently can be seen eosinophilia and thrombocytopenia. Microbiological exams: 1. Thick and thin blood smears – 2. The stain of choice is Giemsa 3. Another method uses fluorescent dye benzothiocarboxypurine P. falciparum antigen detection 4. Dot blot assay 5. PCR amplification: high sensitivity, and rapidity. Diagnosis Malaria is associated with patients having a history of travel within an endemic area. Recent transfusion or intravenous drug addiction are, also, associated with this disease Fever, chills, splenomegaly, anemia Positive thick and thin blood smears Differential diagnosis Malaria can mimic other diseases such as gastroenteritis, pneumonia, hepatitis, meningitis, sepsis, encephalitis. Treatment Antimalarial drugs are classified by the stage of malaria against which they are effective: A. Erythrocytic cycle (schizonts): sulfonamides, sulfones, proguanil, pyrimethamine, colchicine, amodiachine, quinine, quinidine, cloroquine mefloquine, artemisinine; B. Erythrocytic cycle (gametocytes): proguanil, primaquine C. Exoerythrocytic cycle: primaquine, pyrimethamine. Prophylaxis It is indicated to persons who are going into areas where malaria is endemic. For areas free of chloroquine resistant P. falciparum: chloroquine 500mg po/week starting 1-2 wks before travel, during travel, and 4 wks post-travel; for children-5mg/kg/wk once - For areas with chloroquine resistant P. falciparum: mefloquine 250mg po/wk, the same schema or doxycycline 100 mg po daily for adults and children>12 years atovaquone 250mg – proguanil100mg Treatment Cloroquine is the treatment of choice for P. vivax, P. ovale, P. malariae and uncomplicated falciparum malaria (cloroquine sensitive), 1g po (=600mg base), 0,5g at 12 h, 24 and 36 h + primaquine 26mg (15mg base) x 14 days Treatment of cloroquine - resistant P. falciparum: - oral: Quinine sulfate 600mg tid/day + doxicicline 100mg bid x7days/pyrimethamine-sulfadoxine 3tb once on last day of quinine – sulfate - parenteral: Quinine gluconate 10 mg/kg iv over 1-2 h, then 0,02mg/kg/min until start of oral therapy (monitor EKG 72h) (drug used in US) -Quinine dihydrochloride 20mg/kg loading dose in 10-mg/kg 5% glucose over 4 h followed by 10 mg/kg over 2-4 h q8h (max 1800mg/day) until start of oral therapy. TOXOPLASMOSIS Dr. Daniela Leca Toxoplasmosis describes the clinical or pathological disease caused by T. gondii and is distinct from toxoplasma infection, which is asymptomatic in the vast majority of immunocompetent patients. Chronic (latent) infection ensues in all infected people after resolution of the acute phase due to asymptomatic persistence of T. gondii in the cyst form. Reactivation of the chronic infection with resultant toxoplasmosis occurs almost exclusively in patients who are severely immunocompromised. Etiology Toxoplasma gondii is worldwide distributed. Cats (which maintain an enteroepithelial sexual cycle of T. gondii) are the definitive hosts; all other infected animals are secondary hosts (and have an extraintestinal asexual cycle). Three life forms of T. gondii occur: 1. The oocyst 2. The tachyzoite (the asexual invasive form), is seen during the acute infection;. 3. The tissue cyst contains bradyzoites and is able to persist in tissues during the chronic or late phase of the infection. Epidemiology Toxoplasma infection is a worldwide zoonosis. The organism infects herbivorous, omnivorous, and carnivorous animals. The prevalence of tissue cysts in meat used for human consumption is high. Excretion of oocysts has been reported in approximately 1 percent of cats. Pathogenesis and Immunity Pathology The histopathology changes in toxoplasmic lymphadenitis are frequently distinctive and often diagnostic. There is a typical triad of findings: a reactive follicular hyperplasia, irregular clusters of epitheloid histiocytes and focal distension of sinuses with monocytoid cells. Toxoplasma encephalitis (TE) in AIDS patients typically produces brain abcesses with three characteristics zones. The central area is avascular. Surrounding this is an intermediate hyperemic area with a prominent inflammatory infiltrate and perivascular cuffing by lymphocites, plasma cells, and macrophages. Edema, vasculitis, hemorrhage, and cerebral infarction may also be observed. Diffuse TE (DTE) is characterized by widespread microglial nodules without abcesses in the gray matter of the cerebellum, and brain stem. Pulmonary toxoplasmosis may appear in the form of interstitial pneumonitis, necrotizing pneumonitis, and/or consolidation. Chorioretinitis in AIDS patients - segmental panophtalmitis and areas of coagulative necrosis. Myocarditis- focal necrosis with edema and an inflammatory infiltrate. Myositis- necrotic muscle fibers with a variable inflammatory reaction. Extensive involvement of the gastrointestinal tract may occur with variation in the inflammatory response, hemmorhagic gastritis and colitis. Other organs reported to be involved during toxoplasmosis include the liver, pancres, seminiferous tubules, prostate, adrenals, kidneys, and bone marrow. Clinical manifestations Toxoplasmosis is conveniently considered in four categories: (1) acquired in the immunocompetent patient, (2) acquired or reactivated in the immunodeficient patient, (3) ocular, and (4) congenital. 1. Acute acquired toxoplasma infection in the immunocompetent patient 2. Acute Toxoplasmosis in the immunodeficient host 3. Ocular toxoplasmosis in immunocompetent patients 4. Congenital toxoplasmosis Diagnosis Acute infection is diagnosed by: the isolation of Toxoplasma from blood or body fluids by inoculation of specimens into the peritoneal cavities of mice; demonstration of trophozoites in histologic sections of tissue or in cytologic preparations of body fluids; demonstration of characteristic lymph nodes histology; demonstration of Toxoplasma tissue cysts in the placenta, fetus, or neonate. PCR amplification for detection of T. gondii DNA in body fluids and tissues . demonstration of antigen in body fluids by the ELISA technique . Serologic tests for demonstration of antibody: - Sabin-Feldman dye test, ELISA, the IFA test, Differential diagnosis Treatment Chemotherapy of infection with Toxoplasma in pregnant women Chemotherapy through the end of the 15th week of pregnancy: spiramycin 3.0 g (9 mIU) per day in 3 divided doses Chemotherapy from the 16th week of pregnancy onwards - independent of prior treatment with spiramycin, the following therapy should be initiated for four weeks: - Sulfadiazine: 50 mg/kg/d up to 4 g PO in 4 doses - Pyrimethamine: 50 mg on the first day, 25 mg on the consecutive days, PO o.d. - Folinic acid: 10 to 15 mg/d, PO (prevention of inhibition of haemopoiesis) - For monitoring haemopoiesis, weekly blood counts are mandatory. In case of allergic reactions, sulfadiazine administration should be replaced by spyramicin. Chemotherapy in neonates and infants: First, administer for four weeks: Sulfadiaazine: 50-100 mg/kg/d PO in 4 divided doses Pyrimethamine: 2 mg/kg on the first day, 1 mg/kg on the consecutive days PO, o.d. Folinic acid: 5 mg twice a week Corticosteroides: (in the presence of symptoms involving the CNS or the eyes): 1-2 mg/kg/d PO, only until remission of symptoms Thereafter, again for four weeks: Spyramicin: 100 mg/kg/d (300.000 IU/d) in two divided doses These therapy cycles, lasting four weeks each, have to be carried out in the alternation for one year. Monitoring haemopoiesis are required every week. Table 1. Guidelines for acute primary therapy of toxoplasmic encephalitis in patients with AIDS Drug Dosage schedule Standard regimens Pyrimethamine Folinic acid (leucovorin) plus Sulfadiazine or Clindamycin Oral 200 mg loading dose then 50 to 75 mg daily Oral, IV, or IM 10- 20 mg daily (up to 50 mg daily) Oral 1- 1,5 g every 6 hours Oral or IV 600 mg q6h (up to IV 1200 mg q6h) Possible alternative regimens a. Trimethoprim/ sulfamethoxazole b. Pyrimethamine and folinic acid c. Clarithromicyn d. Azythromycin e. Atovaquone f. Dapsone Oral or IV 5 mg (trimethoprim component)/ kg q6h As in standard regimens plus one of the following Oral 1 g q12h Oral 1200- 1500 mg daily Oral 750 mg q6h 0ral 100 mg daily HUMAN IMMUNODEFICIENCY VIRUS INFECTION Egidia Miftode Acquired immunodeficiency syndrome was first reported in 1981 in the Morbidity and Mortality Weekly Report under the title “ Pneumocystis pneumonia – Los Angeles”. In 20 years, the AIDS epidemic has grown from a series of small outbreaks in several risk groups throughout the United States and western Europe into a global public health calamity. Tremendous advances have been made in understanding the molecular mechanisms, in achieving of antiretroviral therapy and blood-supply safety. Although the disease was first encountered in homosexual men andinjectiondrug users, the risk groups soon included transfusion recipients, infants,female sexual contact of infected men, prisoners, Haitians and Africans. Structure of the HIV-1 virion Table 1. Genes and gene products of HIV 1 and 2 Genes Gag Pol Env Vif Vpr Tat Rev Vpu Nef vpx Proteins p17, p24, p1, p2, p6, p7. Protease, RT, integrase gp120, gp41 virion infectivity protein viral protein R transactivator of of transcription regulator of expression of virion protein viral protein U (HIV-1 only) negative regulatory factory virion protein X (HIV-2 only). The life cycle of HIV-1 The major steps in the HIV life are: I. Afferent functions 1. Binding and entry. 2. Reverse transcription, nuclear import, and integration of viral DNA II. Efferent functions Viral transcription (production of mRNA Production of viral regulatory factors Virion assembly. Virus budding and release Pathogenesis of HIV disease The main target of HIV infection is the CD4-positive-lymphocyte population, although infection of tymocytes, macrophages and dendritic cells, can occur. Additional co-receptor which are used for virus entry into CD4-T cells are: 1. CC-chemokine receptor CCR5 2. CXC-chemokine receptor (CXCR4)-. Acute HIV infection Viral load levels in the blood rise; Dissemination of the virions CD4 T-cells count decrease Appearance of HIV-specific CTL (cytotoxic T lymphocytes) which kill infected cells and secrete antiviral cytokines and chemokines). Chronic HIV infection CD4 T-cells number and function continue to decline during the period of clinical latency; Defects in T-cell function/loss of specific responses to recall antigens; Abnormalities in B-cell, natural killer, monocytes and dendritic cell functions Altered cytokine secretion with reduced production of IFN-gamma, IL-2 and IL-12 may occur The virus continue to replicate at all stages of infection Mechanisms of immune depletion are: 1. 2. 3. 4. Direct cytopathic effect Syncytium or multinucleated cell formation, Apoptosis (programmed cell death). Removal of infected CD4 T cells and dendritic cells by the vigorous HIV-specific CTL responses Clinical manifestations Classification of HIV-1 disease into six stages is based on a combination of clinical features and CD4 count: 1. Initial infection (acute seroconversion syndrome) 2. Early HIV-1 disease 3. Intermediate HIV-1 disease 4. Late HIV-1 disease 5. Advanced HIV-1 disease 6. Terminal HIV-1 disease. 1. Initial infection (acute seroconversion syndrome) – usually occurs within 2 to 6 weeks (median=21 days) after exposure to the virus. - may be asymptomatic or - may evolve with symptoms of flu-like or mononucleosis-like illness The most common symptoms are: fever, lymphadenopathy pharyngitis, esophagitis, aphthous ulcerations myalgias, arthralgias headache diarrhea morbilliform skin eruption neurologic manifestations: meningitis, peripheral neuropathy, myopathy, cranial nerve palsies. 2. Early HIV-1 disease - is defined by a CD4 cell count greater than 500cells/mmc. Most persons are asymptomatic. Among symptoms: lymphadenopathy (cervical, axillary and inguinal chains), dermatologic abnormalities: seboreic dermatitis, perifolliculitis, eosinophilic folliculitis, oral lesions: aphtous ulcerations, hairy leucoplakia. 3. Intermediate stage of HIV-1 disease – is defined by a CD4 count between 200 and 500 cells/mmc and could evolves without symptoms or with mild disease manifestations: Recurrent herpes simplex infection Oropharyngeal/vaginal candidiasis Varicella zoster virus infection Weight loss Bacterial sinusitis, bronchitis, pneumonia Headache, myalgias, arthralgias 4. Late-stage disease – is defined by a CD4 cell count between 50 and 200 cells/mmc. These patients have a great risk of developing opportunistic infections such as Pneumocystis carini pneumonia, Toxoplasma gondii infection, cryptosporidiosis, tuberculosis, Kaposi’s sarcoma, esophagial candidiasis and hematologic abnormalities (anemia, neutropenia, idiopathic thrombocytopenia). 5. Advanced HIV disease – is defined as a CD4 count of less than 50 cells/mmc. The most frequent conditions seen during this stage are: MAC disease, cryptococcal meningitis, cytomegalovirus retinitis, progressive multifocal leukoencephalopathy. 6. Terminal HIV disease – symptoms of disease cannot be controlled because no treatment are available The management includes psychological support, family support, and pain management. Classification Table 2. Clinical categories Clinical category A Asymptomatic HIV infection Persistent generalized lymphadenopathy Primary (acute) HIV illness Clinical category B Symptomatic, nonA or C conditions - Candidiasis, oropharyngeal - Candidiasis, vulvovaginal(>1 mo) - Bacillary angiomatosis - Cervical displasia, Clinical category C Candidiasis: esophageal, trachea, bronchi Coccidioidomycosis, extrapulmonary Cryptococcosis, extrapulmonary Cryptosporidiosis>1mo severe, or carcinoma in situ - Constitutional symptoms: fever, diarrhea>1 month CMV retinitis, or in other than liver, spleen, nodes HIV encephalopathy Histoplasmosis, extrapulmonary, disseminated Isosporiasis>1 mo Kaposi’s sarcoma Lymphoma M.avium/kansasii: extrapulmonary M. tuberculosis infection Pn.carinii pneumonia Pneumonia, recurrent Progressive multifocal leukoencephalopathy Salmonella bacteremia, recurrent Toxoplasmosis, cerebral Wasting syndrome HIV encephalopathy Table 3. Classification system CD4 cell category 1) >500/mmc 2) 200-499/mmc 3) <200/mmc CLINICAL CATEGORY A B A1 B1 A2 B2 A3 B3 Pulmonary diseases Any CD4 level: CD4<200/mmc C C1 C2 C3 CD4<100/mmc Cryptococcosis Coccidioidomycosis Histoplasmosis CD4<50/mmc Aspergilosis M. avium Neurologic manifestations Brain involvement A. Focal lesions B. Nonfocal complications Spinal cord Meninges Peripheral nerve and root lymphomatous meningitis; Table 4. Classification of oral lesions: Viral lesions Hairy leukoplakia Herpes simplex Herpes zoster Cytomegalovirus Fungal lesions Candidiasis Histoplasmosis Cryptococcosis Bacterial lesions Periodontal disease Necrotizing stomatitis Mycobacterium avium complex Bacillary angiomatosis Neoplastic lesions Kaposi’s sarcoma Non-Hodgkin’s lymphoma Hodgkin’s lymphoma Autoimmune/idiopathic lesions Salivary gland disease Aphtous ulcers Abnormal pigmentation Dermatologic complications Table 5. Skin lesions Maculopapular lesions Moluscum contagiosum Syphilis Mycobacterial infection Kaposi’s sarcoma Nodular, verrucous, and/or ulcerative lesions Bacillary angiomatosis Cryptococcosis Sporotrichosis MAC infections Kaposi’s sarcoma Vesicular, bulous or pustular lesions Herpes simplex virus Varicella-zoster Cytomegalovirus Staphylococcal impetigo Stevens-Johnson syndrome Papulosquamous lesions Seborrheic dermatitis Dry-skin syndrome Psoriasis Norwegian scabies Staphylococcal folliculitis Eosinophilicfolliculitis Table 6. Acute infectious diarrhea Campylobacter jejuni Clostridium difficile Enteric viruses Enteroadherent E. coli Salmonella Shigella Spectrum of associated malignancies Kaposi’s sarcoma Primary CNS lymphoma Non-Hodgkin’s lymphoma Cervical carcinoma Hodgkin’s disease Seminoma Cryptosporidia Cytomegalovirus Entamoeba histolytica Isospora belli Microsporidia Mycobacterium avium Laboratory tests HIV antibody tests Enzyme immunoabsorbant assay (EIA). Western-Blot (WB). Immunofluorescent assay 1. Rapid detection methods: 2. Home tests kits Viral detection Treatment Table 7. Antiretroviral drugs Antiretroviral drugs Nucleoside reverse-transcriptase inhibitors (NRTI) Group A drugs - Zidovudine (ZDV) - Retrovir - Stavudine (D4T) - Zerit Group B drugs - Didanosine (ddi) – Videx - Zalcitabine (ddC) - HIVID - Lamivudine (3TC) - Epivir Non-nucleoside reversetranscriptase inhibitors (NNRTI) - Nevirapine-Viramune - Delavirdine - Rescriptor Protease inhibitors - Saquinavir - Invirase - Saquinavir - Fortovase - Indinavir – Crixivan - Ritonavir – Norvir - Nelfinavir - Viracept Doses 300 mg bid or 200 mg tid 40 mg bid (>60 kg), 30 mg bid (<60kg) 200 mg bid (>60 kg), 125 mg bid (<60kg) 0,75 mg tid 150 mg bid 200 mg qdx2 wks, then 200 mg bid 400mg tid 600 mg q8h with meals 1200 mg q8h with meals 800 mg q8h, empty stomach 300 mg q12 h-2 wks, then 600mg bid 750 mg q8h Initial regimens Preferred: 2 nucleosides and a PI 2 nucleosides and a NNRTI Under evaluation: 3 nucleosides In patients with CD4<50/mmc or viral load >100 000c/ml: - 2NRTIs + 2 PI - 2NRTIs + PI + NNRTI HAART (highly active antiretroviral therapy) is an antiretroviral regimen that can be expected to reduce the viral load to<50c/ml in treatment-naïve patients. Indications for Antiretroviral therapy Table 8. Indications for the initiation of antiretroviral therapy (DHHS Guidelines-2000) Clinical category Asymptomatic Asymptomatic Acute HIV, or <6 mo after seroconversion Symptomatic (AIDS, unexplained fever) CD4 cell count/HIV RNA CD4<500/mmc, or RNA>10000 (bDNA), or >20000 (RT-PCR) Recommendation CD4>500/mmc and HIV RNA<10000 (bDNA), or <20000 (RT-PCR) All Delay therapy and observe , or treat All Treat Treat Treat Table 9. Management of opportunistic infections in patients with HIV infection Preferred regimen(s) Alternative regimen(s) Pentamidine Pneumocystis carinii Trimethoprim -Acute infection (TMP)15mg/kg/day + 4mg/kg/dayIVx21 sulfamethoxazole days 75mg/kg/day x 21 Clindamycin 600mg days IV + primaquine 30mg base/day Atovaquone 750mg suspension po TMP + dapsone 100mg po/day -Prophylaxis when Trimethoprim/sulfam Dapsone CD4<200mmc, or etoxazole Atovaquone unexplained fever>2 Aerosolized wks pentamidine 300mg q month Candida - Oropharyngeal Clotrimazole 10 mg Fluconazole 100mgpo 5x/day Amphotericin B oral Nistatin 500000u suspension gargled 5x/day Itraconazole Maintenance Fluconazole 100mg 200mg/day po 3x/wk - Esophagitis Fluconazole 200mg/d Ketoconazole x 2-3 wks 200mg/d po Itraconazole Prophylaxis Not recommended Cryptococcal meningitis -Initial treatment Amphotericin 0,7-1 Fluconazole 400-800 mg/kg/d IV +/mg/d po +/flucytosine 100 flucytosine 100 mg/kg/d po x 14 days mg/kg/d x 6-10 wks then fluconazole 400 mg/d x 8-10 wks -Maitenance Fluconazole 200 Amphotericin B 0,6-1 mg/d po mg/kg/d 1-3 wks -Prophylaxis when CD4<100/mmc Fluconazole 200 mg/d po Itraconazole 400 mg po/d Toxoplasma gondii encephalitis -Acute infection -Suppressive therapy -Prophylaxis when positive toxo IgG + CD4<100/mmc Mycobacterium tuberculosis -Treatment -Prophylaxis (if PPD>5mm induration, high-risk exposure) Mycobacterium avium complex (MAC) -Treatment -Prophylaxis (if CD4<50/mmc) Pyrimethamine 100- Pyrimethamidine + 200mg loading dose clindamycin 900then 50-100 mg/d po 1200mg >6wks + sulfadiazine 4-8 g/d Azithromycin 1800 at least 6 wks + mg/d 1st day then folinic acid 1200 mg/d x 6wks, Pyrimethamine 25-75 then 600mg/day mg po + sulfadiazine 0,5-1 g/d TMP Dapsone 50mg/d+ sulfamethoxazole pyrimethamine 50mg/wk -no concurrent use of PI/NNRTIs INH/RIF/PZA/EMB INH/RIF daily or 2-3 daily x 2 mo x/wk x 18 wks -concurrent PI/NNRTIs INH/RFB/PZN/EMB INH/RFB daily or daily x 8 wks 2x/wk x 18 wks INH/SM/ PZN/EMB INH/SM/PZA 2-3/wk daily x 8wks x30 wks INH 300 mg/d x 9mo Rif 600 mg/d + PZN 20 mg/kg/d x 2 mo FQ/PZN or EMB/PZN (for multiply-resistant strain) Clarythromycin 500 Azithromycin 600 mg bid + EMB+/mg/d + EMB+/rifabutin 300 mg/d rifabutin Amikacin/ciprofloxac in Rifabutin 300mg, po Clarythromycin 500 qd mg bid Cytomegalovirus retinitis -Treatment Initial Maintenance -Prophylaxis (when positive CMV serology + CD4<50/mmc) Cryptosporidia Isospora Microsporidiosis Herpes simplex - mild - severe Azithromycin 1200mg q wk Ganciclovir 5 mg/kg IV bid x 14-21 days Foscarnet 60 mg/kgq8h x 14-21 d Cidofovir 5mg/kgIV x 2 then 5mg/kgq 2wks Foscarnet 90-120 mg/kg/d Ganciclovir 5 mg/k/d Ganciclovir-oral 1g tid Paromomycin 2 gx 14-28 days TMP/sulfametox. x 24wks Albendazole 400800mg >3wks Nitazoxanide 1 g Azithromycin Pyrimethamine 50-75 mg/d x 1 mo Metronidazole 1,5 g Acyclovir 400mg tid, Famciclovir Acyclovir 800 mgpo 5x/d >7 days Valacyclovir 1g tid Foscarnet 40 mg/kg IV q 8h 3 wks SEPTICEMIA, SEPSIS, SEPTIC SHOCK Egidia Miftode Definition An expert consensus conference of the American College of Chest Physicians and the Society of Critical Care coined the phrase systemic inflammatory response syndrome (SIRS) to describe a clinical syndrome believed to be the result of an overly activated inflammatory response. This new definition recognized the important role that endogenous mediators of systemic inflammation play in sepsis, which was no longer regarded as being caused by microbial pathogenicity factors alone . Septicemia is a dramatic clinical syndrome, which result from acute invasion of the bloodstream by certain microorganism or their toxic products. Fever, chills, tachycardia, tachypnea and altered mentation are common acute manifestations of septicemia. Bacteremia: is the presence of vivid bacteria in bloodstream confirmed by the blood culture with the isolation of a pathogen. Systemic inflammatory response syndrome (SIRS): contains two or more of the following conditions: 1. fever, over the 38oC or hypothermia less than 36oC; 2. tachypneea more than 20 breaths/min; 3. tachycardia more than 90 beats/min.; 4. leucocytosis more than 12000/mmc; 5. leucopenia less than 4000/mmc or more than 10 percents immature forms. - Sepsis means: SIRS plus a documented infection Severe sepsis Septic shock Multiple organ dysfunction syndrome (MODS) Respiratory: PaO2<80 mmHg, PaCO2>50mmHg, respiratory rate<5/min, or >50/min; Renal system: increased serum creatinine, oliguria (<480ml/24h); Cardiovascular system: hypotension, heart rate<55/min, ph<7.25; Hepatic system: serum bilirubin>60mg/l, TP<15%; Gastrointestinal system: bleedings, pancreatitis, ileus, perforation; CNS: Glasgow coma score <6; Hematologic system: platelet count<20000/mmc, leucocytes<1000/mmc, hematocrit<20%. Etiology Multiple components of the microbial structure may initiate the systemic inflammatory response. Gram positive: Streptococcus pneumoniae Staphylococcus aureus/epidermidis Streptococcus pyogenes Clostridium Gram negative: Neisseria meningitidis Gram negative bacilli: E. coli, Salmonella, Klebsiella, Pseudomonas, Yersinia pestis, Vibrio vulnificans, Aeromonas species Rickettsia rickettsii Capnocytophaga canimorsus Erlichia species Bartonella species Dengue viruses Viruses - causes of the hantavirus pulmonary syndrome Plasmodium falciparum Babesia microti Anaerobes: peptococcus, microaerofili streptococci, Actimomyces israelii. Epidemiology Predisposing factors include: diabetes mellitus, cirrhosis, alcoholism, leukemia, lymphoma or disseminated carcinoma, cytotoxic chemotherapy and immunosuppresive drugs which cause neutropenia, total parenteral nutrition, a variety of surgical procedures and infections arising from the urinary, biliary or gastrointestinal tracts. Pathogenesis and pathology Most of the bacteria causing gram-negative sepsis are normal commensals in the gastrointestinal tract. From there they may spread to contiguous structures (as in peritonitis after appendiceal perforation), or they may migrate from the perineum into the urethra or bladder. Gram-negative bacteremia follows infection in a primary forms, usually the: genitourinary tract, biliary tree, gastrointestinal tract or lungs and less commonly, the skin, bones and joints. Metastatic abscess formation may complicate bacteremia. The involved target organs are: lungs: pulmonary-edema, hemorrhage and hyaline membrane formation, abscesses, bronchopneumonia kidney: tubular or cortical necrosis, myocardium: patchy necrosis, superficial ulceration or even hemorrhage necrosis gastrointestinal tract, superficial ulceration or even hemorrhage necrosis capillaries in many tissues: leukocyte-platelet or fibrin thrombus formation brain /meninges: abscesses, meningitis Pathophysiology Table 1: Virulence factors of Staphylococcus aureus and their proposed pathogenic mechanism VIRULENCE FACTORS OF STAPHYLOCOCCUS AUREUS AND THEIR PROPOSED PATHOGENIC MECHANISM Twart host defenses - Microcapsule Protein A Coagulase Fatty acid-metabolizing enzyme Leukocidin and/or gama-toxin Invade tissue Proteases Nucleases Lipases Hyaluronate lyase Staphylokinase Elicit sepsis syndrome Toxic shock syndrome toxin Enterotoxins Cytolytic toxins (alpha, beta, gama and delta) Induce specific toxinosis Toxic shock syndrome toxin Enterotoxin Exfoliative toxin Attach to endothelial cells and basement membrane Binding proteins for fibrinogen, fibronectin, laminin, collagen, vitronectin and thrombospondin Clinical manifestations Clinical manifestations are related to the following elements: I. Portal of entry Skin: erysipelas, staphylococcal skin infection, trauma, burns; Respiratory tract infections Gastrointestinal tract lesions: stomatitis, gingivitis, teeth extraction, enterocolitis; Genito-urinary tract infections II. Lymphangitis and primary sites of infection III. Positive blood cultures: hematogenous dissemination is manifested with fever, chills, headache; IV. Metastatic foci of infection: Cardiovascular system: infective endocarditis, myocarditis, pericarditis Central nervous system: brain abscess, epidural abscess, purulent meningitis (confusion, obtundation, coma); Respiratory system: bronchopneumonia, pleural effusion (acute respiratory distress syndrome, tachypnea, hypocapnea); Gastrointestinal: liver and spleen abscess, impaired gastrointestinal motility, sterss related mucosal disease, hyperbilirubinemia, elevation of liver enzymes. Renal system: renal abscess, nephritis Bone and joint involvement: osteitis, osteomyelitis, spondylodiscitis, arthritis Cutaneous manifestations: cellulitis, flegmons, diffuse erythroderma (caused by gram-positive organisms and by the action of pyrogenic or erythrogenic toxins); colorful skin lesions such as ecthyma gangrenosum (associated with P. aeruginosa septicemia), colorful vezicular or bullous lesions, cellulitis, petechial lesions (may appear in gram negative septicemia) Toxic shock syndrome Clinical manifestations 1. Hemodynamic changes:. 2. Dermatologic findings: 3. Severe myalgias, muscle tenderness, weakness 4. Diarrhea, nausea, vomiting] 5. Encephalopathy 6. Respiratory distress syndrome 7. Acute renal failure 8. Hepatic necrosis 9. Disseminated intravascular coagulation. Table 4: Streptococcal Toxic Shock Syndrome Streptococcal Toxic Shock Syndrome – definition Symptoms Signs An acute, febrile illness that begins with a mild viral-like prodrome or minor softtissue infection and may progress to shock, multiorgan failure and death Early symptoms are vague: Viral-like prodrome Severe pain and erythema of an extremity Mental confusion Hypotension, systolic Laboratory features Fever > 38 degree Soft-tissue swelling Tenderness Respiratory failure, rales, cyanosis, tachypneea Hematologic: Marked left shift Decline in hematocrit Thrombocytopenia - Renal azotemia (2,5 x normal on admission) and hematuria - Hypocalcemia - Hypoalbuminemia - Creatine phosphokinase elevation - Pulmonary abnormalities: -Pulmonary infiltrate on chest x-ray -Hypoxia Table 5. Laboratory investigations in sepsis Complete blood count Blood chemistry Urinalysis Chest radiography Erythrocyte sedimentation rate Blood cultures (3 or more separate specimens) Cultures from other biologic fluids (pus, pleural effusion, joint effusion, catheter, etc) Differential diagnosis Infectious diseases Bacterial infections Rickettsial infections: Viral infections:, Parasitic infections:; Fungal infections:. Non-infectious diseases A. Neoplastic diseases:; Antinuclear antibodies Rheumatoid factor Computed tomography of abdomen, pelvis, other sites Radionuclide scans Venous duplex imaging of lower limbs Echocardiography B. C. D. E. Autoimmune diseases: Hypersensitivity; Granulomatous diseases: Other: CNS hemorrhage, CNS degenerative diseases, familial Mediteranean fever, cholangitis. Treatment A. Initial therapy for the sepsis syndrome Community-acquired infections 1. Suspected staphyloccocal etiology: oxacillin+aminoglycoside 2. Suspected genito-urinary source: a third generation cephalosporin (3GC); a quinolone; ticarcillin/piperacillin with/without an aminoglycoside; 3. Non-urinary tract source: 3GC or ampicillin/ticarcillin/piperacillin – beta lactamase-inhibitor (BLI) Hospital-acquired infections: Non-neutropenic patients: 3GC with/without metronidazole, cefepime, a beta lactam drug-BLI/imipenem with/without an aminoglycoside Neutropenic patients: ceftazidime + an aminoglycoside; ticarcillinclavulanat/piperacillin-tazobactam + an aminoglycoside; imipenem/meropenem + an aminoglycoside. Tabel 6.First intention treatment and alternative therapy for infective endocarditis on native valve Etiology 1. S.aureus meti-S First intention treatment 1. (oxaciline 8g/d iv, every 4h)x4-6wks +gentax35days 2. S.aureus meti-R 2. (Vanco 2g/d iv, every 12h)x4-6wks 3. S.viridans, S. bovissensitive to Pen. G(CMI<o,1microg/ ml) 3. Pen.G 12-18milU/d, iv, cont/4hx2 wks + genta 3mg/kg/d x2wks or Pen G x4wks or Alternative 1. Cefazolin 6g ivx46weeks+genta3mg/ kgx3-5d, OR 2. Vanco 4-6wks 3. (Ceftriaxone + genta)x2wks Ceftriaxone 4g/d x4wks 4. S.viridans, S.bovis with CMI PenG:0,10,5microg/ml 4. Pen G 18mil U/d x4wks+genta 3mg/kg/d x 2wks, 5. .S viridans, S.bovis (CMI PenG>1), enterococi sensitive to ampi/Pen G, vanco, genta 6. Enterococi (CMI Pen G >16ng/ml) 7. Enterococi pen/ampi R, high resistance to genta, vanco-R 8. Difteroizi sensitive to genta (MIC<4/ml) 9. Difteroizi resistent to genta (>4/ml) 10.gram-negative cocobacili from the group HACEK* 5. Pen G 18milU/24h, 46spt+genta 4-6wks, or 5. (vanco+genta) (AMP12g/zi iv cont or x4-6wks every 4h+genta)x4-6wks 11.Bartonella 11.Fluoroquinolone or RIF or macrolide 12.Amfotericine B+/-an azol (fluconazole) 13.Vanco+RIF+gentax6wks 12.Candida 4. vanco 30mg/kgc/d until la 2g/dx4wks 6. Vanco+gentax4-6wks 7. Quinupristin/dalfopristin or linezolid 1200mg/zi iv 7.Vancox6wks 8. PenG+gentax6wks 9. Vancox6wks 10.Ceftriaxone 2-3g iv or cefotaxime 3g ivx6wks 10.Ampi 12g/d+(strepto 15mg/kg-1g/d or genta)x6wks 13.EI on valvular prothesis with negative bloodcultures Genta=gentamicine, RIF=rifampicine, HACEK include Haemophilus sp, A.actinomycetemcomitans, Cardiobacteriu.hominis, Eikinella corrodens, Kingella sp. Table 7. Infective endocarditis on prosthetic valve Etiology First intention treatment Alternative S. epidermidis S.aureus meti-R S.aureus meti-S (Vanco 2g/d iv, every 12h+RIF 900mg/d, every 8h)x6wks +genta* 240mg/d every 8 hx2wks “ “ “ (oxaciline 8g/d iv, every 4h+RIF)x6wks +gentax2wks Vanco+Fluoroquin olone+RIF “ “ “ Vanco+RIF+gentax6wks EI on valvular prothesis with negative bloodcultures As for the native valve For the other etiology- endocarditis. B. Adjunctive measures in the treatment of sepsis syndrome 1. Maintenance of adequate tissue perfusion with volume replacement: normal saline solution, fresh frozen plasma, albumin, dextran, crystaloid solutions. 2. Sympatomimetic amines: dopamine, dobutamine 3. Corticosteroids for gram-negative rod septicemia-is controversed. 4. Diuretics for the early oliguric phases of schock. LYME DISEASE (LYME BORRELIOSIS) Dr. Daniela Leca Lyme disease, originally called Lyme arthritis, was recognized in 1975 because of a close geographic clustering of affected children in Lyme, Connecticut, who were thought to have juvenile rheumatoid arthritis. The illness is now known to be a multisystem disorder caused by the ticktransmitted spirochete, Borrelia burgdorferi Etiology Borrelia burgdorferi grows best at 33 oC in Barbour-Stoenner-Kelly medium. Epidemiology Lyme disease is now the most common vector-borne infection in the USA and also occurs in Europe, Scandinavia, the former Soviet Union, China, Japan, Australia. Pathogenesis B. burgdorferi may survive primarily in certain niches within the joints, nervous system, or skin where may persist, latently or symptomatically, for years. Clinical manifestations Stage 1 (localised infection, erythema migrans) Erythema chronicum migrans (ECM) . Stage 2 (disseminated infection) ECM Neurologic symptoms - Within several weeks after the onset of illness, about 5 percent of untreated patients develop cardiac involvement: Stage 3 (persistent infection) Frank arthritis occurs primarly in large joints, especially the knee, usually one or two joints at a time. ECM, begins with red violacious lesions that become sclerotic or atrophic. These lesions, may last for many years. Sclerotic lesions may look like localized scleroderma. -chronic neurologic manifestations as subacute encephalopathy (with increasing cerebrospinal fluid protein levels or evidence of intrathecal antibody production to B. burgdorferi), encephalomyelitis, encephalitis, dementia, psychiatric syndromes, brain stem abnormalities, extrapyramidal syndromes. The Laboratory Diagnosis 1. Culture of the spirochete from patient specimens permits a definitive diagnosis, Serologic (ELISA), indirect immunofluorescence assay (IFA), antibodycapture immunoassay (EIA), Western blotting or immunoblotting. In neuroborreliosis, comparison of the antibody response to B. burgdorferi in CSF and serum by EIA is a helpful diagnostic test (a CSF/serum ratio of specific antibody of greater than 1 is suggestive of intrathecal antibody production). The nonspecific laboratory abnormalities are: - a high erythrocyte sedimentation rate, - an elevated serum IgM level, - an increased serum glutamic-oxaloacetic transaminase (SGOT) level, glutamic-pyruvic transaminase (SGPT) and lactate dehydrogenase (LDH). - anemia early in the illness - occasionally elevated WBC counts with shifts to the left in the differential count - microscopic hematuria, sometimes with mild proteinuria - C3 and C4 levels are generally normal or elevated - tests for rheumatoid factor or antinuclear antibodies are usually negative. Treatment Lyme disease can be treated with oral antibiotic therapy, except for neurologic abnormalities, which require intravenous therapy. For patients with infection localized to the skin, 10 days of therapy is generally sufficient. For patients with disseminated infection, longer courses of 20–30 days are recommended. Approximately 15 percent of patients with early disseminated infection experience a Jarisch-Herxheimer-like reaction during the first 24 hours of therapy. In vitro, B. burgdorferi is sensitive to tetracycline, penicillin, macrolides and to third-generation cephalosporins, but it is resistant to rifampin, ciprofloxacin, and aminoglycosides. Reinfection may occur in patients who are treated with antibiotics early in the illness. With any of the recommended treatment regimens, treatment failure may occur and retreatment may be necessary. Prevention The risk of tick bites in high-risk areas can be reduced by simple measures, particularly by wearing long trousers tucked into socks and by checking for ticks after exposure in wooded areas. INFECTIONS CAUSED BY LISTERIA MONOCYTOGENES Daniela Leca Human disease due to L. monocytogenes generally occurs in the setting of pregnancy or immunosupression due to illness or medication and is characterised by invasive syndromes such as meningitis, sepsis, chorioamnionitis and stillbirth. Epidemiology Listeria monocytogenes has been isolated from soil, dust, food products for humans, both animal and vegetable, animal feed, water, sewage, and almost every type of animal cultured, including asymptomatic humans. Pathogenesis Listeria monocytogenes can invade the eye and the skin of humans after direct exposure, or may cross the placenta during maternal bacteremia and infect the placenta, amniotic fluid, and fetus. Clinical Syndromes The results of an infection with L. monocytogenes can be manifest in a number of five different syndromes. The most common result of contact with the organism appears to be a transient, asymptomatic carrier state. Infections in Pregnancy These infections may occur any time during pregnancy, but more often in the third trimester. The patient usually complains of chills and fever and back pain. Granulomatosis Infantiseptica is caused by transplacental transmission Sepsis of Unknown Origin Meningoencephalitis Cerebritis Focal Infections 1. Skin infections 2.Ocular infections: purulent conjunctivitis, nonpurulent conjunctivitis, acute anterior uveitis with isolation of the organism from the anterior chamber. 3. Lymph node infections 4. Subacute bacterial endocarditis 5.Other focal infections include arthritis, osteomyelitis, prosthetic joint infection, spinal or brain abscess, peritonitis, cholecystitis, acute hepatitis. Diagnosis Treatment Ampicillin or penicillin appears to be the best drugs. Experimental laboratory results show synergism between either ampicillin or penicillin and aminoglycosides against L. monocytogenes. Trimethoprim-sulfamethoxazole (15/75mg/kg per day, intravenously, in three divided doses) has been used in penicillin-allergic patients . Vancomycin is a possible alternative, Prognosis Treatment of maternal bacteremia during pregnancy can prevent neonatal infection. Antibiotic therapy for the newborn can limit sequelae, although the widely disseminated disease characteristic of granulomatosis infantiseptica is frequently fatal regardless of treatment. Mortality varies considerably according to the syndrome. Granulomatosis infantiseptica and meningitis in immunosuppressed patients have the highest mortality, ranging in the former from 33 to 100 percent and in the latter from 12.5 to 43 percent. HEMORRHAGIC FEVER Hemorrhagic fever viruses are a group of RNA viruses, which have rodent hosts, and, some of them have arthropod vectors, but other are transmitted from person to person. Yellow fever virus Yellow fever virus is an RNA virus that belongs to the family Flaviviridae. Epidemiology The disease occurs in tropical America and in Africa in endemic zones (15 degrees north latitude and 15 south latitude). Epidemic (urban) Yellow fever is transmitted by Aedes aegypti mosquitoes (after feeding on viremic humans, they may transmit infection for 1-2 weeks). In South America - only the sylvatic cycle of transmission, involving forest mosquitoes and monkeys-occurs. Sporadic cases and outbreaks- occur during the late rainy season when vector ( Hemagogus mosquitoare) is most abundant. In yellow fever may occurs a cross-immunity to dengue and other flaviviruses. Pathophysiology necrosis of hepatocytes, Councilman bodies in the sinusoides degeneration/necrosis of the myocardium tubular necrosis on the kidney depletion of lymphocytes B (that may compromise the immune response). Clinical features Incubation period is 3-6 days Yellow fever evolves as a biphasic illness progressing through three stages: 1. Infection – is characterized by abrupt onset: fever chills, headache, backache, myalgias, nausea and vomiting flushing of the face and conjunctivae, relative bradicardia; 2. Remission – occurs after 3-4 days (duration of hours to 1-2 days) 3. Intoxication - symptoms recur and intensify: jaundice renal dysfunction hemorrhagic diathesis encephalopathy (because hepatic or renal failure). Table 1. Transmission of viral Hemorrahagic Fever diseases agents Transmitted by: Disease Mosquitoes Dengue hemorrhagic Rift Valley fever Yellow fever Crimean-Congo hg. Fever Kyasanur and Omsk hg fever Argentine HF(Junin virus) Bolivian HF(Machupo v) HF with renal syndrome Lassa fever Tick Rodents Unknown Etiologic agent family Togaviridae Etiologic agent genus Flavivirus Bunyaviridae Togaviridae Bunyaviridae Phlebovirus Flavivirus Nairovirus Togaviridae Togaviridae Arenaviridae Flavivirus Flavivirus Arenavirus Arenaviridae Arenavirus Bunyaviridae Hantavirus Arenaviridae Marburg and Ebola Filoviridae virus diseases Filoviridae Arenavirus Filovirus Filovirus Complications: bacterial infections Laboratory diagnosis leukopenia albuminuria prolonged prothrombin time elevation of ALAT and bilirubin(direct and indirect) +/-thrombocytopenia hypoglycemia serological tests (fourfolds increases in dynamic) in hemagglutination inhibition(HI), complement fixing(CF), neutralizing antibodies IgM (EIA)-rapid test histopatologic examination which reveals Differential diagnosis: malaria, dengue, influenza, leptospirosis, typhoid fever, viral hepatitis hemorrhagic fevers produced by: Lassa, Ebola, Marburg, CongoCrimean, and Rift-Valley fever viruses certain intoxications. Prevention: yellow fever vaccine for ages 9-12 months Dengue The dengue syndrome is an acute febrile viral disease characterized by exanthem, headache, myalgia, anorexia, gastrointestinal disturbances, and prostration caused by an RNA virus (transmitted by mosquitoes, principally A. aegypti). Epidemiology Between 250000-500000 cases of dengue hemorrhagic fever, dengue shock syndrome (DHF/DSS) occurs throughout the world annually. Countries principally involved are China, the Philippines, Vietnam, Laos Cambodia, Thailand Malaysia, Cuba, and, sporadically India, Bangladesh, Sri Lanka. Pathogenesis Clinical manifestations Dengue fever Clinical features are different in children and in adults. Infants/young children: pharyngeal inflammation, rhinitis, mild caugh (lasts for 1-5 days); In adults the disease is characterized by: incubation period: 1-7 days sudden onset with fever, back pain, macular, generalized rash, relative bradicardia followed by: myalgia/ arthralgia, nausea, vomiting, generalized lymphadenopathy, hyperesthesia, taste aberration after defervescence—a morbilliform, maculopapular rash appears (1-5 days duration), followed by descuamation. Dengue hemorrhagic fever has a biphasic evolution: -the first phase is mild: malaise, vomiting , headache -the second phase (after 2-5 days) – with rapid clinical deterioration and collapse, gastrointestinal bleeding, ecchymosis, flushed face, irritability, hepatomegaly. Laboratory findings increase in hematocrit value trombocytopenia leukocytosis prolonged bleeding time fibrin split products are elevated ALAT are moderately elevated metabolic acidosis increased urea nitrogen chest X ray shows pleural effusions in all patients Virus isolation-from blood Serologic diagnosis (ELISA, FA, CF, HI, N test)-I sample<5days of illness -II sample >2weeks after the onset Differential diagnosis Dengue fever must be distinguished from: Influenza – like diseases, viral respiratory tract infections Early stage of malaria Hepatitis leptospirosis diseases Chikungunya virus (in Africa-arthralgia), West Nile virus four arboviral diseases with dengue-like courses, but without rash: Colorado tick fever, Rift Valley fever, Ross River fever, sandfly fever; Dengue hemorrhagic fever: rickettsial diseases, meningococcemia other hemorrhagic fevers, and other severe illness with hemorrhagic manifestations Treatment is supportive (bed rest, antipyretic drugs, etc) If shock: replacement of fluid and electrolytes Transfusions of fresh blood or of platelets suspended in plasma. Simptomatics: cloral hydrate, diuretics, digitalis therapy. Prophylaxis consists of avoiding mosquitoes bites and vaccination. Arenaviruses and Hemorrhagic Fever (HF) 1. Lassa fever Geographic repartition: Nigeria, Liberia, Sierra-Leone, Guinea; Transmission: rodent urine; human-human via infected secretion Incubation: 7-17 days; Delay until major signs appear: 7 days after the onset; Symptoms: fever, headache, joints pain, non-productive cough Day 5: pain on deglutition, pharyngeal exudat, vomissment, diarrhea; Day 7: face edema, purpuric and petechial eruption; bleeding is associated with fatal outcome. Complications: liver involvement, encephalopathy, pericarditis, pleural effusion, ascites, MOD. Laboratory diagnosis: Lymphopenia, proteinuria, elevated blood urea nitrogen; specific IgM appear early Treatment-Convalescent-phase plasma is effective for arenaviruses hemorrhagic fever, and Ribavirin. Fatality rate: 15-20%. 2. Junin virus and Machupo virus HF Geographic repartition: Argentine (for Junin virus), and Bolivia (for Machupo virus); Transmission: rodent-human via urine Clinical aspects are similar for both diseases: -unlike Lassa fever, respiratory symptoms, sore throat and encephalopathy are infrequent. -among the most prominent manifestations are: conjunctivitis, erythema of the face, neck and thorax. The same treatment is indicated as for Lassa Fever. Bunyaviridae and Hemorrhagic Fever 1. Rift Valley HF Etiology: an ARN virus-Phlebovirus (belongs to Bunyaviridae) Geographic repartition: W and S- Africa, Egypt Incubation period: 3-4 days Delai until major signs appear: 2-4days after the onset Symptoms: like Dengue - abrupt onset, biphasic fever, headache, myalgia, arthralgia, face congestion, chemosis; Complications- blinding, encephalitis, convulsions, hemiplegia Laboratory diagnosis-blood antigen, presence of IgM (ELISA), funduloscopic examination is suggestive; Reservoir: sheep, cows Vector-mosquitoes Inter-human contamination is possible. Treatment: symptomatic, vaccination, Interferon, Ribavirin. 2. Crimean-Congo HF Etiology: Nairovirus. Geographic repartition: S-E Europe, Chine, S, E-Africa, W, Central Sahara Transmission: tick bite, contact with blood of humans/domestic animals; Incubation period - 5-12 days Delai until major signs appear: 3-6 days after the onset Symptoms - fever, headache, myalgia, edema of the neck, sleepness, conjunctivitis, pharyngitis Complications-cerebral bleeding, renal and liver failure, Laboratory diagnosis – presence of IgM antibodies Reservoir: like Rift Valey HF Vector: Hyalomma tick 3. HF with renal syndrome Etiology: Hantavirus (Hantaan, Seoul, Puumala, Dobrava) Geographic repartition: worldwide (also in France) Transmission: Aerosols from rodent excreta; Incubation period: 7-15 days Delai until major signs appear: 3-6 days after the onset Symptoms: trouble of vision, abdominal pain, hypotension, bradicardia, proteinuria, shock, renal failure, bleeding. Complications: melena, thrombocytopenia Laboratory diagnosis: presence of specific antibodies. Vector: yellow neck mouse/or bankvole. Treatment: specific anticorp, dialysis 4. Hantavirus with pulmonary syndrome Etiology: Hantavirus sin nombre; Symptoms: fever, shock, pulmonary edema; Vector: deer mouse; Filoviridae and HF Marburg/Ebola virus diseases Geographic repartition: S-Africa, Kenya, Zimbabwe, Sudan, Zaire, Congo, Gabon; Pathogenesis of Ebola virus infection Incubation: 5-10 days Delay until major signs appear: 5 days after the onset Abrupt onset with: fever, chills, headache, lumbar pain, myalgia; abdominal pain, nausea, vomiting, diarrhea, ulcerations of the lips and tongue, pharyngitis. rash of the face, trunk, arms; bleedings (>50% of patients) CNS involvement: somnolence, delirium, coma; Complications: liver and kidney involvement, pancreatitis, After a week of evolution: defervesces or death in shock; Laboratory diagnosis: detection of antibodies by IF on infected cells, culture of virus from blood, urine, or throat swab. Only laboratories with the highest level of safety (P4 facilities) are allowed to handle these viruses. Reservoir/Vector: There is abundant circumstantial evidence to indicate that the natural habitat of the two filoviruses is the tropical rain forest. It seems that, in the wild, the virus circulates between small mammals and highly specialized insects, both dwelling either predominantly or exclusively in the canopy of the rain forest. According to this scenario, small arboreal mammals would be the actual reservoir of the virus, and blood sucking insects its vectors. Route of transmission: blood exposure, or contact with body fluids possibly by aerosols. Treatment – is symptomatic. Mortality in Marbourg infection is 25% and in Ebola infection is 80-90%.