Spring Break Homework Handout
Spring Break Homework: Nuclear and Organic Chemistry
Sections to Read: 21.1, 21.2, 21.4, 21.6, 24.2, 24.3, and 24.4
Every year, there are a few multiple choice questions concerning the topics of nuclear and organic chemistry.
While they are not a major portion of the exam, the questions on these topics are relatively easy, so knowledge of this content area will increase your success on the exam! Please read the assigned sections (Chapter 21 and
24 in textbook) and complete the problems that follow. There will be a quiz on this after spring break!
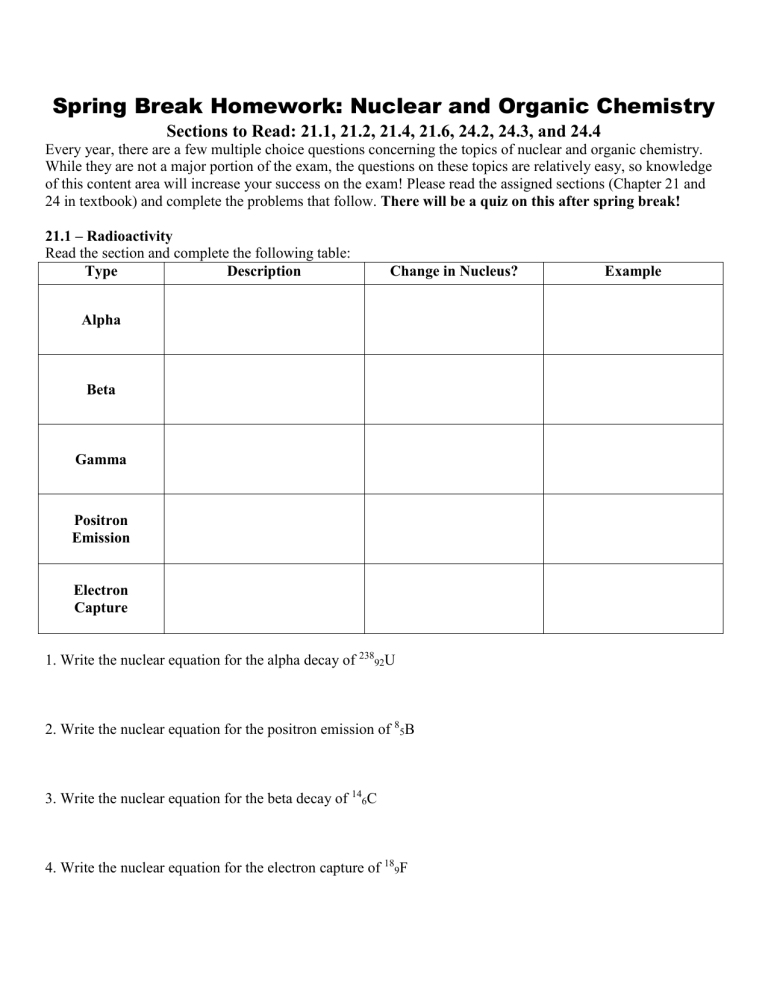
21.1 – Radioactivity
Read the section and complete the following table:
Type Description Change in Nucleus? Example
Alpha
Beta
Gamma
Positron
Emission
Electron
Capture
1. Write the nuclear equation for the alpha decay of
238
92
U
2. Write the nuclear equation for the positron emission of
8
5
B
3. Write the nuclear equation for the beta decay of
14
6
C
4. Write the nuclear equation for the electron capture of 18
9
F
21.2 – Patterns of Nuclear Stability
Read the section and answer the following question:
This section discusses the importance of nuclear stability in relation to proton to neutron ratios. Below, please draw the graph that indicates the number of protons to the number of neutrons. Additionally, explain why this graph can be used to predict the type of decay that a molecule undergoes.
21.4 – Half Lives
Read the section and answer the following questions:
What order are all nuclear decay reactions?
Because all nuclear reactions are this order, what is the integrated rate law for this order reaction?
How do we calculate ½ lives for this order of reactions? What is a ½ life?
5. After 44 minutes, a sample of 44
19
K is found to have decayed to 25% of the original amount present. What is the half-life of
44
19
K?
6. Neon-23 is an unstable nuclide with a half-life of 38 second. (a) What form of decay would neon-23 be expected to undergo? Write the balanced nuclear reaction for this process. (b) If you had 52 g of Neon-23, then how much would remain after 4 minutes?
21.6 – Energy Changes in Nuclear Reactions
Read this section and answer the following questions:
What is the equation to relate energy and mass?
If mass is lost during a reaction, then what is this mass converted into? Why must we calculate this for nuclear reactions?
What are nuclear binding energies? How are they calculated? How are they different from typical nuclear reactions?
7. The following alpha decay is observed:
238
92
U
4
2
He +
234
90
Th
The masses of the nuclei are uranium = 238.0003 amu, Th = 233.9942 amu, and He = 4.0015 amu. Calculate the amount of energy released in this reaction.
8. You know that: mass of proton (mp) = 1.007276 amu, mass of neutron (mn) = 1.008665 amu, and mass of electron (me) = 5.485799 × 10 -4
amu. Calculate the mass defect (in amu) and nuclear binding energy (in
J/nucleon) for Carbon-12 (nuclear mass: 11.996708 amu)
24.2 – Introduction to Hydrocarbons
Read this section and answer the following questions
Define what alkanes, are:
MEMORIZE the following prefixes for alkanes:
What are structural isomers? Give an example!
24.3 – Alkenes, Alkynes, and Aromatic Hydrocarbons
Read the section ( Do NOT worry about addition reactions and substitution reactions) and answer the questions:
Define what alkenes, alkynes, and aromatic hydrocarbons are:
What are geometric isomers? Give an example!
24.4 – Functional Groups
Be able to name and identify the following functional groups
9. Name the following hydrocarbons: a. C
2
H
6
: ___________________ b. CH
3
CH
2
CHCH
3
: ___________________
c. d.
: __________________________
: _________________________ e.
: ___________________________________
Isomers
10. What is the relationship between each of the following pairs of structures? Are they totally different molecules (i.e., which do not have the same molecular formula), are they isomers , or are two drawings of the same compound?
NOTE – The solid lines indicate an atom coming out of the page at you and a dashed line indicates a line going back into the page!





