Atomic Structure Worksheet: Protons, Neutrons, Electrons
advertisement
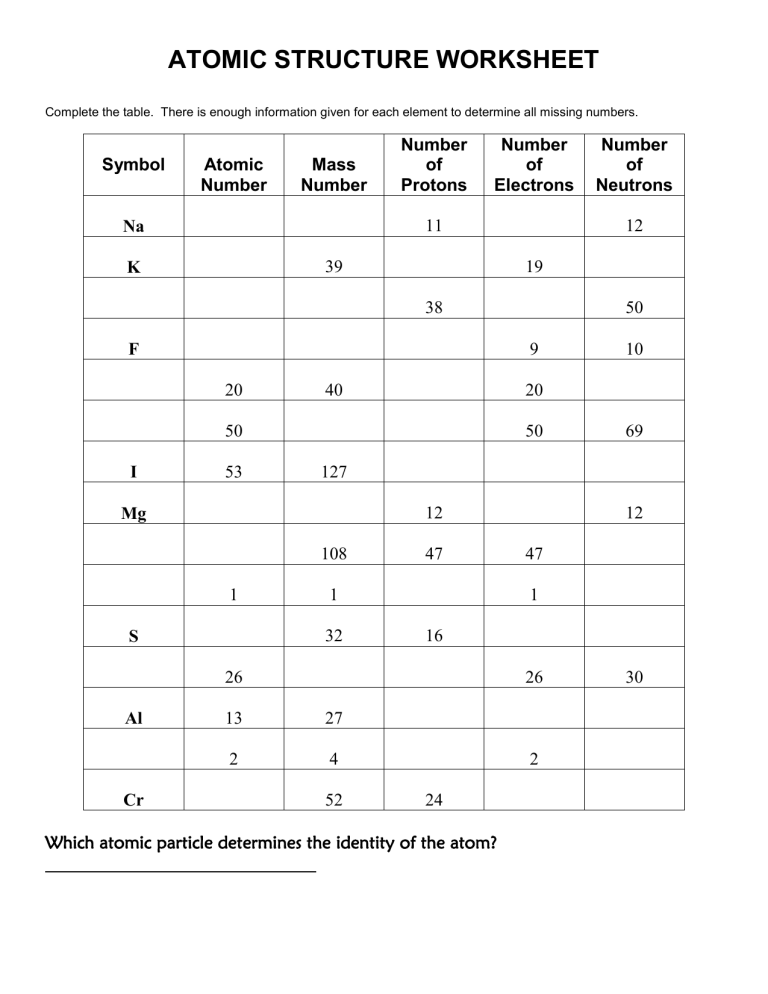
ATOMIC STRUCTURE WORKSHEET Complete the table. There is enough information given for each element to determine all missing numbers. Symbol Atomic Number Mass Number Number of Protons Number of Electrons 11 Na 39 K 12 19 38 40 53 50 12 108 47 1 32 S Cr 12 47 1 16 26 Al 69 127 Mg 1 10 20 50 I 50 9 F 20 Number of Neutrons 26 13 27 2 4 52 2 24 Which atomic particle determines the identity of the atom? 30 Atomic Structure (Use your notes to answer the following) are the building block of all matter! Matter is anything that has a and takes up . All atoms are made up of smaller subatomic particles called 1 2 3 . Even though these particles are so small that we can’t see them with our eyes, they still have masses called units. Each particle has it own mass. For example protons and neutrons have an AMU of but electrons are 2000 x’s smaller so their AMU is . Every atom has an atomic number which tells you the number of or . Atoms also have an , which tells you the number of neutrons and protons. The cool thing about these subatomic particles is that they all have their own charge! Protons have a charge, Electrons have a charge and since Neutrons are neutral, so they have charge. Because of their charges, these subatomic particles are only found in one of two areas in the atom. and are found in the nucleus while electrons are found in the . Since all of the electrons are found in the electron cloud, it has a overall charge. While all the protons and neutrons in the give it a positive overall charge. In every atom, there are an equal number of protons and electrons, so the overall charge of any atom on the periodic table is . This is because the and charges cancel each other out! Scientists use models to help us understand an atom’s structure. Bohr models are made up of a nucleus and rings, orbits or . Electrons in the outermost shell of an atom are called . These electrons are the most important electrons because they will determine if an atom will bond or not! An atom can have a maximum of shells. The first shell can hold a maximum of electrons, second shell electrons, third shell electrons, fourth shell electrons, fifth shell electrons, sixth shell electrons and the seventh shell holds electrons. Atoms make up elements. Elements cam from distant . Dmitri Mendeleev first organized the elements on a chart which we now call the periodic table of elements. They are arranged according to the atom’s . The periodic table itself is split up into two sections. The left side of the periodic table are and the right side are the . The element’s atomic symbols that you see on the table are the abbreviations of their Latin names.




