2.3.1 Enthalpy Changes Booklet 2011_2012
advertisement
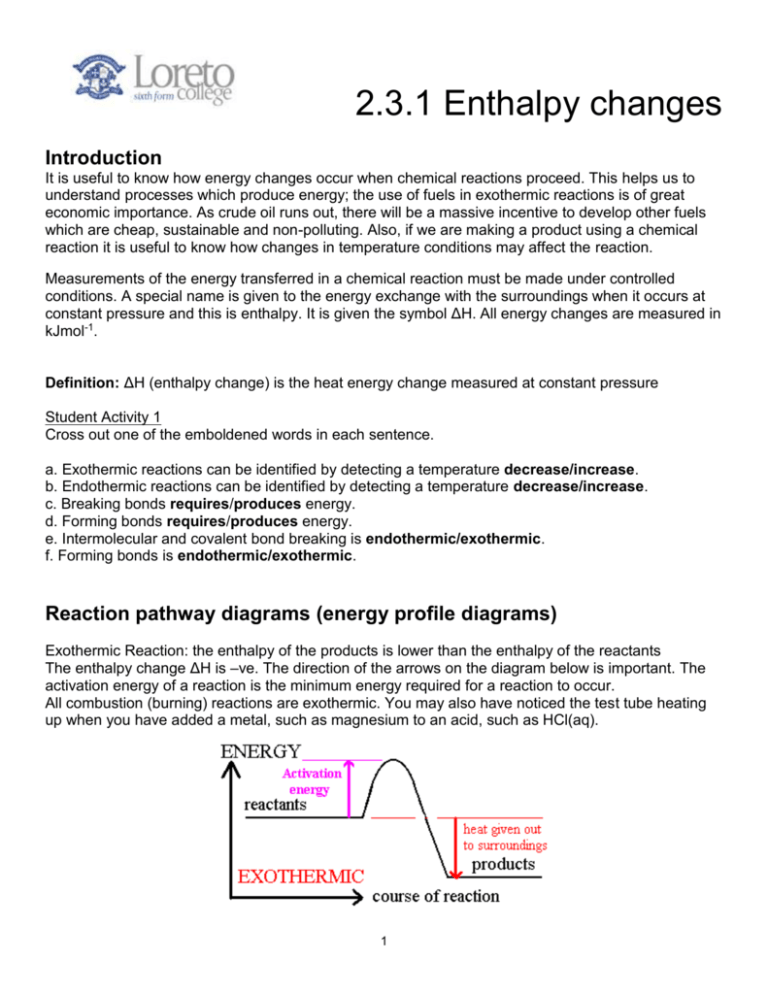
2.3.1 Enthalpy changes Introduction It is useful to know how energy changes occur when chemical reactions proceed. This helps us to understand processes which produce energy; the use of fuels in exothermic reactions is of great economic importance. As crude oil runs out, there will be a massive incentive to develop other fuels which are cheap, sustainable and non-polluting. Also, if we are making a product using a chemical reaction it is useful to know how changes in temperature conditions may affect the reaction. Measurements of the energy transferred in a chemical reaction must be made under controlled conditions. A special name is given to the energy exchange with the surroundings when it occurs at constant pressure and this is enthalpy. It is given the symbol ΔH. All energy changes are measured in kJmol-1. Definition: ΔH (enthalpy change) is the heat energy change measured at constant pressure Student Activity 1 Cross out one of the emboldened words in each sentence. a. Exothermic reactions can be identified by detecting a temperature decrease/increase. b. Endothermic reactions can be identified by detecting a temperature decrease/increase. c. Breaking bonds requires/produces energy. d. Forming bonds requires/produces energy. e. Intermolecular and covalent bond breaking is endothermic/exothermic. f. Forming bonds is endothermic/exothermic. Reaction pathway diagrams (energy profile diagrams) Exothermic Reaction: the enthalpy of the products is lower than the enthalpy of the reactants The enthalpy change ΔH is –ve. The direction of the arrows on the diagram below is important. The activation energy of a reaction is the minimum energy required for a reaction to occur. All combustion (burning) reactions are exothermic. You may also have noticed the test tube heating up when you have added a metal, such as magnesium to an acid, such as HCl(aq). 1 Endothermic Reaction: the enthalpy of the products is higher than the enthalpy of the reactants. The enthalpy change is +ve. These reactions are less obvious in the real world. Examples include: photosynthesis which involves the combination of carbon dioxide and water to form glucose; the decomposition of carbonates; cool packs used on the football pitch. Student Activity 2 Classify the following as either endothermic or exothermic. Reaction (example) Oxidation (combustion of CH4) Thermal decomposition (CaCO3 → CaO + CO2) Respiration Acids reacting with metals (Li + HCl → LiCl + ½ H2) Photosynthesis Endothermic or exothermic Student Activity 3 a) Complete the following sentences with the words endothermic or exothermic. A student was monitoring the temperature in three different reactions. The first reaction increased in temperature by 4.5 °C. This is an ............................. reaction. The second reaction went from 25 °C to 12 °C. This is an .......................... reaction. The third reaction changed in temperature from -5.8 °C to -3.4 °C. This is an ........................... reaction. b) Draw enthalpy profile diagrams for the first reaction and the second reaction. 2 Standard conditions The size of the enthalpy change of a particular chemical reaction depends upon: temperature; pressure; physical state; amount of reactants. All energy changes therefore must be measured under the same conditions. This is known as standard conditions. Definition: standard conditions are:- temperature = 298 K pressure = 1 atm or 100kPa solution concentration = 1 moldm-3 all substances should be in their standard sates at 298K and 100kPa Any enthalpy changes measured under standard conditions are termed a standard enthalpy change H 298 The definitions of the different types of standard enthalpy changes The standard enthalpy of reaction ( H r) The heat energy change at constant pressure when the amounts of reactants shown in the equation react under standard conditions to give the products in their standard states. Example: The standard enthalpy of formation ( H f) The enthalpy change when 1 mole of a compound is formed in its standard state from its constituent elements in their standard states, under standard conditions. Example: The standard enthalpy of combustion ( H c) The enthalpy change when 1 mole of a substance in its standard state reacts completely with oxygen under standard conditions. Example: Bond enthalpy ( H BE) The enthalpy change when 1 mole of a given type of bond is broken by homolytic fission in the gaseous state. Example: Student Activity 4 a) Write in the names and symbols of the energy changes next to the correct definition. Energy change Definition The enthalpy change when 1 mole of a given type of bond is broken by homolytic fission in the gaseous state. The heat energy change at constant pressure when the amounts of reactants shown in the equation react under standard conditions to give the products in their standard states. The enthalpy change when 1 mole of a substance in its standard state reacts completely with oxygen under standard conditions. The enthalpy change when 1 mole of a compound is formed in its standard state from its constituent elements in their standard states, under standard conditions. 3 Symbol b) Link the reactions to the enthalpy definition that best fits it. One is completed for you. The standard enthalpy of formation 6C(s) + 6H2(g) → C6H12(l) 2H2(g) + N2(g) → N2H4(l) ½F2O(g) → F(g) + ½O (g) C(s) + 2H2(g) + ½O2(g) → CH3OH(l) H2(g) + ½O2(g) → H2O(l) The standard enthalpy of combustion ¼CH4(g) → ¼C(g) + H(g) Na(s) + ¼O2(g) → ½Na2O(s) Bond enthalpy 2Na(s) + ½O2(g) → Na2O(s) CH4(g) + 2O2(g) → CO2(g) +2H2O(l) 4 Note: Mean bond enthalpy is the average energy required to break and separate one mole of bonds of one type occurring in a range of compounds/molecules. The mean bond enthalpy will never correspond exactly to the actual bond enthalpy. Though not required, it is useful to know about the enthalpy of neutralisation (see experiment 13). Student Activity 5 Label the following statements as either true or false a) Boiling water is endothermic. b) The following reaction represents the enthalpy of combustion of ethane. 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g) c) The formation of a molecule of nitrogen molecules from nitrogen atoms will be accompanied by a temperature decrease. d) The bond enthalpy of a C-H bond in methane is the same as that of a C-H bond in methanol. e) The reaction between nitric acid and sodium hydroxide will be expected to have a negative ∆H sign. Experimental methods for calculating standard enthalpies of reaction When experiments have been carried out the enthalpy change can be calculated using: Where:Q = Heat Change m = Mass of substance heated c = Specific Heat (for water = 4.18 J g-1 K-1 T = Change of temperature Specific heat capacity is defined as the quantity of energy required to heat 1g of substance by 1K. 5 Calculating the enthalpy of neutralisation, Hn Definition: The standard enthalpy change of neutralisation ( H n) is the enthalpy change when one mole of water molecules is formed when an acid reacts with an alkali at 298K and 100kPa. The solutions must have a concentration of 1moldm-3 example: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l) The apparatus for the experiment is shown in Experiment 13. Example Calculation 3 250cm3 ( 0.4moldm-3 ) of NaOH was added to 250cm ( 0.4moldm-3) of HCl. The temperature rose from 17.4oC to 20.1oC. Assume that the density of the solution is the same as that of water (=1gcm -3) Assume the density of the solutions = density of water Mass of solutions = 500g c = The specific heat capacity of water is 4.18J g-1 K-1. Q = m c T Equation: NaOH (aq) + HCl(aq) → NaCl (aq) + H2O (l) …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. 6 Calculating the enthalpy of combustion, HC The apparatus for the experiment is shown in Experiment 14. A more accurate way to find this will be demonstrated by your teacher. Student Activity 6 Past paper question (Jun 2009 Q2b) 7 Calculating enthalpy of solution Definition: The energy change at constant pressure when 1 mole of solid is completely dissolved in excess water under standard conditions. In this experiment the temperature is measured over time and a graph is plotted. Extrapolation of the curve obtained as the solution cools allows correction to be made to the estimated temperature rise. This corrected value makes an allowance for losses to the surroundings. The temperature of the liquid is measured at the start of the practical. This is recorded. The solid is added and the temperature of the liquid is recorded every 30 seconds. There will either be a rise or fall in the tempearture of the liquid. A typical set of results is given below: Mass of water = 100g Mass of solid (sodium hydroxide) = 6.20g Initial temperature = 15oC Results Recorded Time (s) Temperature (oC) 0 15 30 21 60 28 90 29 120 27 150 26 180 26 210 26 In order to find the actual temperature rise a graph of temperature against time is plotted. Plot time (horizontal axis) against temperature from 15 oC to 30 oC and extrapolate from two straight lines to get the maximum temperature without cooling. 8 Calculation …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. Using Hess’s Law to calculate enthalpy changes Some enthalpies cannot be found by direct experiment. Instead we have to find these enthalpies from other known enthalpies and the method of drawing energy cycles and using Hess’s law. There are two different methods which depend on the type of data provided: a) Finding the enthalpy of formation from enthalpies of combustion and b) Finding the enthalpy of reaction from the enthalpies of formation. Definition; Hess's Law (a consequence of the first law of thermodynamics- energy can not be created or destroyed) A B C HAB = HAC + HCB 9 a) Finding the enthalpy of formation from enthalpies of combustion Calculate the enthalpy of formation of methane given the enthalpies of combustion of methane, carbon and hydrogen. → CH4(g) Hf = ? CH4(g) + 2O2(g) → CO2(g) + 2H2O (g) H1= -890.4 kJ mol -1 C (s) → CO2(g) H2= -393.5 kJ mol -1 → H2O (g) H3= -285.7 kJ mol -1 C(s) + 2H2(g) Combustion Equations + O2(g) H2(g) + ½ O2(g) We can use Hess' Law to calculate the enthalpy of formation of methane from the enthalpy of combustion data. Constructing the Energy Cycle 1. Write the equation for the enthalpy we need to calculate. Make the top arrow long. Hf C(s) + 2H2(g) CH4 (g) 2. At the bottom, write the combustion products of carbon dioxide and water which are common to both sides of the equation and draw in the arrows to make a triangle. 3. Add the values 4. Applying Hess' Law to calculate the missing value on the top equation. 10 b) Finding the enthalpy of reaction from the enthalpies of formation Calculate the enthalpy of reaction for: NH3 (g) + HCl (g) → NH4Cl(g) Hr=? Given the enthalpies of formation of ammonia and hydrogen chloride. NH3(g) HCl(g) NH4Cl(g) -46.0kJ mol-1 -92.3kJ mol-1 -315kJ mol-1 Construction of the energy cycle 1. Write the reaction for the enthalpy we need to calculate Hr NH3 (g) + HCl (g) NH4Cl(g) 2. Add the elements for formation which are common to both sides of the equation 3. Complete the triangle by adding the values and arrows 4. Applying Hess Law will calculate the value as required Quick Method For finding HӨr when given HӨf you can use this little shortcut: HӨr = Σ HӨf products - Σ HӨf reactants Try it with the examples above and see if it works. But beware – you cannot use this formula in any other circumstances. 11 Finding the enthalpy change in a reaction using bond enthalpies If enough energy is absorbed by atoms bonded together in a molecule they vibrate so violently that the bond breaks. The energy required to break these bonds is called the bond enthalpy (or bond energy). In examinations these energies will always be given. Bond H-H H-Cl C-C (single) C-H C-O C-Cl C=C (Double) C≡C (Triple) Cl-Cl Br-Br H-Br N≡N (Triple) H-N O=O O-H C=O Bond Enthalpy / kJ mol-1 +436 +431 +347 +413 +335 +326 +619 +837 +242 +193 +364 +945 +391 +498 +464 +805 When bonds are made energy is released. The process is exothermic, H is negative. When bonds are broken energy is needed. The process is endothermic, H is positive. Definition: bond enthalpy is the energy needed to break one mole of bonds in the gaseous state by homolytic fission. Definition :mean bond enthalpy is the average energy needed to break one mole of bonds in the gaseous state by homolytic fission. Example Calculate the approximate enthalpy of reaction for the following process: CH2=CH2 + H2 CH3-CH3 Bonds Broken Bonds Formed Calculate ΔHr as the difference between the energy needed to break the old bonds and the energy given out when the new bonds are made. This is the sum of bonds broken (which is always positive) and bonds formed (which is always negative). 12 Draw an energy profile for this reaction. Student Activity 7 Calculate the enthalpy of the combustion of methane using mean bond enthalpies. Draw an energy profile diagram. 13 Why do these values not agree exactly with accurate experimental values? Remember that most experimental values for finding heats of combustion have big heat losses so there you always get a smaller value than the book value. Extension Questions 1. How would the value for the enthalpy of combustion of an alcohol where the steam is allowed to condense to room temperature compare with that where the steam is kept in the gaseous state? …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. 2. It is very difficult to determine the standard enthalpy change of formation of hexane directly. Suggest a reason why. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. …………………………………………………………………………………………………….. 3. 14 4. Complete the following table by including definitions and symbols Energy change Standard enthalpy of combustion Definition Symbol Standard enthalpy of formation Standard enthalpy of reaction Standard bond enthalpy 15