Staining a root tip and calculating its mitotic index Teacher/lecturer
advertisement

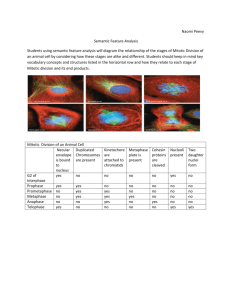
Staining a root tip and calculating its mitotic index Teacher/lecturer guide Type and purpose of activity This experiment can be used to: provide evidence for assessment of Outcome 3 develop knowledge and understanding of the process of mitosis develop problem solving skills and in particular Outcome 2 PCs: (b) information is accurately processed, using calculations where appropriate (d) experimental procedures are planned, designed and evaluated appropriately Background information In this activity students will prepare and stain root tips. To achieve an Outcome 3 students must either have TWO different sources of root tips OR stain one type of root tip with two different stains. A comparison between EITHER the root types OR the stains will then be possible. This practical is best carried out in the morning to get good results. Two recommended sources of roots are garlic and hyacinth. The garlic cloves, bought normally for cooking purposes, will produce roots at any time of year. Hyacinth bulbs can be bought at Garden Centres during autumn and winter. Both garlic cloves and hyacinth bulbs will produce ample roots for the experiment. Suitable stains for studying the stages of mitosis in root tips are lactopropionic orcein and toluidene blue. The mitotic index is the fraction of cells in a microscope field which contain condensed chromosomes. This index will be calculated for each slide prepared. Preparation of the plant materials and the stains is covered in the Technical Guide. To make this activity non-seasonal, it is possible to ‘fix’ the root tips when available and then store them until required. Fixing of root tips is only covered in the Technical Guide. Classroom management Students are asked to mark the root tip one or two days prior to staining the root tips. This will enable them to link rate of growth with mitotic index. Microscopic examination of the slides: Students should examine several slides and calculate the mitotic index for each one. Prepared slides could also be available. Science & Plants for Schools: www.saps.org.uk Staining a root tip and calculating its mitotic index: p. 1 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012. Supply of materials In order to satisfy the core skill in problem solving, students will be required to identify and obtain resources required for themselves. Further advice on supply of material is given in the Technical Guide. Extension work Try to vary the mitotic index of the plant tissue e.g. cutting the root tips and keeping them at 0°C for 24 hours may increase the mitotic index. The experimental method can be varied e.g. varying the temperature or concentration of acid; varying the time the root tip is in the acid; squashing the root tip with a coverslip instead of macerating; varying the age of the root used; preparing the stains differently (e.g. different dilutions, different pHs); heating the lactopropionic orcein slide gently; investigating a possible link between rate of growth of root and mitotic index. Acknowledgements Information and advice from Dr Kwiton Jong, Royal Botanic Garden, Edinburgh, is gratefully acknowledged. Information was also received from Ashby Merson-Davies, Sevenoaks School, Kent. This experiment was produced by the SAPS Biotechnology Scotland Project. Funding for the project was provided by SAPS, Unilever and The Scottish Office. Support was also provided by Edinburgh University, Quest International, the Scottish CCC, the Higher Still Development Unit and SSERC. Science & Plants for Schools: www.saps.org.uk Staining a root tip and calculating its mitotic index: p. 2 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012. Technical Guide The class will be varying EITHER plant material OR stain for this activity. The list of materials required will vary depending on this decision. Materials required Materials required by each student/group: gloves and eye protection compound microscope (x100 - x400 magnification) small beaker of 1 M hydrochloric acid (2 will be required if plant material is being investigated) small beaker of water and dropper microscope slides coverslips fine forceps dissecting needle scissors soft tissue paper ruler fine thread dropping bottle of lactopropionic orcein AND/OR (see below) dropping bottle of toluidine blue garlic clove with suitable roots AND/OR (see below) hyacinth bulb with suitable roots Materials to be shared: water bath at 60°C marker pen timer dropping bottle of 50% glycerol dropping bottle of 70% ethanol lens tissue Preparation of materials If PLANT MATERIAL is to be varied prepare BOTH plant types below. If STAIN is to be varied prepare just any one ofthe plant types. To prepare hyacinth bulb roots: Place the bulb in a suitably sized container with water so that the root end is just incontact with the water. It is best to change the water daily if possible. Roots of a suitable length (26 cm) will beavailable within a week and perhaps sooner. CARE! Hyacinth bulbs can cause allergies. Wear gloves if handling the bulbs regularly. Science & Plants for Schools: www.saps.org.uk Staining a root tip and calculating its mitotic index: p. 3 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012. To prepare garlic clove roots: Carefully peel the cloves and place in holes in a foam or polystyrene float. Sit the float in a beaker of water so that the base of the clove is in contact with the water. Roots of a suitable length (26 cm) will be available after 2-4 days. If STAIN is to be varied prepare BOTH stains, as detailed next. If PLANT MATERIAL is to be varied prepare just any one of the stains. CARE! - Wear gloves and eye protection when handling the stains. Lactopropionic orcein should be prepared in a fume cupboard or well ventilated room. Dilute it to a 45% solution by volume with distilled water. Toluidine blue is harmful if swallowed. Prepare a 0.5% solution in a citrate/phosphate buffer at pH4 (20 cm3 0.1 M citric acid + 10 cm3 0.2 M disodium hydrogen phosphate + 8 cm3 distilled water). Fixing the roots This stage is required only if suitable roots are available but they are to be stained at a later date. Mix 6 cm3 absolute alcohol with 2 cm3 glacial acetic acid in a fume cupboard. This mixture is called Farmer’s fluid and must be freshly prepared. Once added to the Farmer’s fluid, the root tips can be stored for many months in a refrigerator. Supply of materials It is not appropriate to provide all equipment and materials in, for example, a tray system for each student/group. Equipment and materials should be supplied in a way that students have to identify and obtain resources. Normal laboratory apparatus should not be made available in kits but should generally be available in the laboratory. Trays could be provided containing one type of specialist equipment or materials. 1. Toluidine blue stain can be purchased in powder form from suppliers such as Philip Harris Education [tel: 01543 480077, fax: 01543 480068]. Make up a 0.5% solution in McIlvaine buffer [0.1M citric acid, 0.2M sodium hydrogen phosphate at pH4]. This should keep for many months at room temperature. 2. Ideal dropping bottles can be purchased from chemists. These are the ones used for eye drops. They are very strong and have a built in dropper. 3. Lacto-propionic orcein stain can be purchased from Philip Harris Education [tel: 01543 480077, fax: 01543 480068] as Orcein Propionic C7A69849 100ml £10.10 (2006) Science & Plants for Schools: www.saps.org.uk Staining a root tip and calculating its mitotic index: p. 4 This document may be photocopied for educational use in any institution taking part in the SAPS programme. It may not be photocopied for any other purpose. Revised 2012.