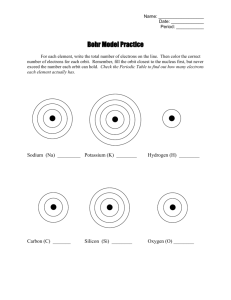
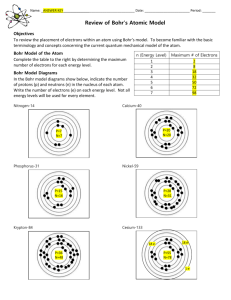
Matter and Energy The electrons located in the outermost energy
advertisement

Matter and Energy The electrons located in the outermost energy level of the atom are called valence electrons. Generally, elements located in the same column on the Periodic Table have the same number of valence electrons. The vertical columns on the Periodic Table are called groups, or families. The Bohr Model and Valence Electrons Recall that atoms are composed of small, subatomic particles. The protons and neutrons are located in the nucleus, while the electrons are located outside the nucleus in the electron cloud. It is impossible to determine the exact location of electrons within the cloud at any particular moment. In order to study atoms, we use models designed by scientists. One of the most common models is the Bohr model, developed by Niels Bohr in 1913. The Bohr model is a simple model that displays some basic information about atoms. Atoms do not actually look like the Bohr model. Like all models, the Bohr model has its own limitations. Although the Bohr model has limitations, the model is often used to study and explain the behavior of atoms. In a Bohr model, electrons are shown orbiting the nucleus in set paths called energy levels. Each energy level can hold only a certain number of electrons. On the Periodic Table, the number of energy levels is the same as the row numbers. These horizontal rows are called periods. Generally, the following rules can be used to make a Bohr model for the first 18 elements on the Periodic Table. The first energy level is located closest to the nucleus and can hold no more than two electrons. The second energy level can hold no more than eight electrons. Lower energy levels must be filled before going to the next energy level. The pattern of electrons filling the energy levels becomes more complex for elements that have an atomic number greater than 18.