Transpiration Lab: Potometer Experiment

IB Biology / IHS
Transpiration: Measuring it with a potometer and serological pipette
Adapted from: AP Biology Lab Manual (1996) and the American Biology Teacher (1998).
Overview: In this lab, we will measure transpiration – the evaporation of water from a plant through its stomata – under different laboratory conditions. Stomata must be open to allow carbon dioxide to enter the leaf for photosynthesis. However, if too much water is lost through transpiration, the plant will wilt and die. Therefore, plants must maintain a balance between CO
2 stomata.
gain and water loss by regulating the opening and closing of their
After doing this lab, you should be able to describe
• the effects of environmental variables on rates of transpiration for one species of plant, using a controlled environment,
• the structures used by plants to transport water and regulate water movement,
• the role of transpiration in the movement of water within a plant,
• how water moves from roots to leaves in terms of the properties of water and differences in water potential.
Materials
1.0 mL serological pipette, plant cutting* w/ 14-18 small leaves or 8-10 large leaves, ring stand, clamps, tubing, tub of water, jar of Vaseline™. Note: Consider bringing gardening gloves! Some leaf margins have S H A R P points!
Steps for making a potometer
1. Obtain and submerge the following in a tub of tap water: pipette, rubber tubing, plant cutting.
2. Just before going to step 3, make a fresh cut at a 45-degree angle across the bottom of the cutting while it is under water.
This minimizes the introduction of air bubbles into the xylem. Plan the cut so the end of the stem just fits the diameter of the rubber tubing.
3. Now connect plant, water-filled tubing, and water-filled pipette. Taking data will be easier if you insert the tapered TIP of the pipette into the rubber tubing.
4. Remove the plant/tubing/pipette from the tub. Bend the tubing into a “U” -shape and clamp to a ring stand so that both pipette and plant are vertical. See Figure 1. You have just constructed a potometer.
5. Check: √ … that you don’t have cut-off petioles or stems; water can leak from them.
√ … that there are no air bubbles in the tubing; the end of the plant must directly contact the water in the tubing.
(As a LAST resort, you can apply Vaseline™ to make an airtight seal around the stem and rubber tubing. BUT, do NOT get any Vaseline™ on the cut end of the stem, or you risk plugging the xylem!)
6. Set up a 60W lamp about 30 cm away from the potometer to warm the leaves and help keep their stomata open.
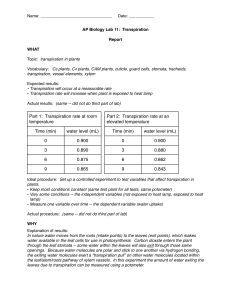
7. Let the potometer equilibrate for 10 min.
8. Record pipette water levels every 15 min for 2-3 h or until the potometer stops working.
9. When you dismantle the potometer, save the plant cutting! a) Cut off all leaves, blot off excess water, and mass them. Record.
Fig. 1 b) Carefully cut out and determine the mass of a 4-cm include the central vein. If you can’t get 4 cm 2
2 section of leaf. Do not
, try for 2 cm 2 . Record. c) Use (a) and (b) to estimate your potometer’s leaf surface area in cm 2 convert to m 2 . Show all calculations!
. Then,
10. Do a preliminary data analysis a) Make a line graph of cumulative water loss per square meter of leaf surface
(mL/m 2 ) as a function of time for your potometer.
*We have used American holly, English laurel, and viburnums; with our potometers, the chosen plant species needs to have a strong stem that resists crushing.
b) Calculate transpiration rate in mL/m 2 •hour from a particular time interval on the graph during which your potometer is functioning and data are roughly linear.