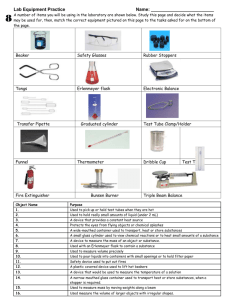
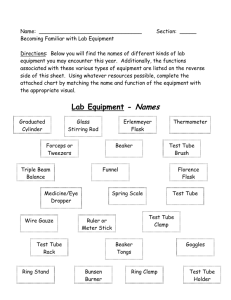
Experiment 12 Estimation of Acid Value, Iodine Value and
advertisement

Experiment 12 Estimation of Acid Value, Iodine Value and Kttstorfer Value 1. Aim of experiment (1) Understood the preparation principle and estimation method of three values. (2) Predominated the conception, application and purpose of three values. 2.Principle of experiment Acid Value: Neutralizing the free acid of 1g raw materials waste the number of KOH. Kttstorfer Value: Neutralizing the hydrolysis acid of 1g raw materials waste the number of KOH. CH 2 O CH 2 O CH2 O O C R O C R' O C R'' + 3NaOH CH 2 O NaO CH 2 O + NaO CH2 O Na O O C R O C R' O C R'' Iodine value: It wastes the number of I2 that 100g oil generates addition reaction with I2. The estimation method of iodine value is weishi method. The course of reaction is the following formulary: R CH CH R + I2 R I I CH CH R 3. Main apparatus and chemical reagents Beaker, conical flask, burette, counter balance, prolong, water bath pot, iodine flask and suction pipet. Potassium hydrate standard solution, phenolphthalein, alcohol- xylene mixture, sodium hyposulfite standard solution, starch indicator, KI, methyl chloroform and chlorhydric acid. 4. Experiment procedures (1) Estimation of acid value. Taking two samples about 3~5g put into two conical flasks. The 50ml alcohol- xylene mixture is put into conical flasks by agitation. Three drop phenolphthalein is put into, and then titrates with potassium hydrate standard solution until solution color become red. Calculate the result. (2) Estimation of iodine value. Taking two samples put into two iodine flasks. The methyl chloroform is put into iodine flasks by agitation. Take 20ml KCl -ice vinegar mixture truly by suction pipet, cover the plug at once. The 20ml KI solution and 100ml water are put into iodine flask after standing 1h. Titrate with 0.1mol.L-1 sodium hyposulfite standard solution. When solution color will become red, 3ml starch indicator is put into, then titrate until solution color become colorless. Bland test is done. Calculate the result. (3) Estimation of kttstorfer value. Taking two samples with about oil 50~60 drops and then putting into two conical flasks. 25ml KOH-alcohol mixture is boiled 1h. Three drops phenolphthalein is put into, and then titrates with chlorhydric acid standard solution until solution color becomes colorless. Bland test is done. Calculate the result. 5. Matters need attention (1) No wasting at standardization. (2) Ethyl ether and alcohol are flammability and volatilization. 6. Questions (1)Which factors mainly affect the speed of Kttstorfer reaction? (2)Which chemical compounds can disturb the results when estimate ester through Kttstorfer reaction? Experiment 17 Preparation of Shampoo 1. Aim of experiment (1) Understood the preparation principle of shampoo product. (2) Known the action of compositions in formulation. 2.Principle of experiment Shampoo is the product of keeping hair clean, which can avoid wiping out nature sebum of hair. So shampoo is both eradicator and luster agent of hair. The kinds of shampoo contain clarity one, pearl light one, cream one, conditioning one, neutron-one, oil one, dryness one and so on. Shampoo can avoid soap dirty. But one question must pay attention is shampoo can’t irritate man’s eyes and skin. Shampoo must have the following points:(1)good decontamination, (2)suitable viscosity, (3)enough foam, (4)luster and facility tease,(5)security high. The shampoo consist of decontamination foam agent, foam stabilizer, thickening agent, sterol, chelant, pearling agent, antiseptic, essence, pigment. Decontamination foam agent: Anion consist of K12, LST, AES, TA-40, AESM, AOS, AGA and PL-1 emulsifier. Ambisexuality consist of BS-12, BS-12K, DCM and OA-12. Non-ion consists of alkanol amide, AEO, APO and TWeen-20. Foam stabilizer consists of OA-12 and alkanol amide. Thickening agent consists of NaCl, NH4Cl, fatty acid polyethylene glycol ester, amine oxide and colloid which increase shampoo’s consistency. Clarifier consists of alcohol, propanediol and fatty alcohol citric acid ester which can keep shampoo lucid. Sterol consists of oil, fat, higher alcohols and ester which keep hair lubricity and luster. Chelant consist of EDTA, EDTA-2Na and EDTA-4Na which can fall deposition of calcium and magnesium ion on the hair. Pearling agent consist of glycol stearic acid ester, carbowax glycol stearic acid ester, cetanol and stearyl alcohol which can bring Pearling light. Antiseptic consist of methylparaben and third ester mixture and so on. Antioxidant consists of BHT, BHA and vitamin E which can prevent shampoo oxidation. Essence and pigment. 3. Main apparatus and chemical reagents Beaker, motor agitator, thermometer, microscope, counter balance, electric heater and water bath pot. AES, OP-10, ABS-Na, BS-12, lanolin, citric acid, essence and pigment. 4. Experiment procedures (1)Water is put into 250ml beaker. The beaker is heating to 60℃. (2) AES is put into 250ml beaker and keep temperature 60℃ until melted. (3)Other surface agents are put into 250ml beaker until melted. Assistant is put into 250ml beaker. (4)Fall and put essence and antiseptic into 250ml beaker. (5)Adjust pH value to 5.5-7.0. (6)Adjust viscosity by salt. AES Other agent surface Heating Essence Adjust pH value Adjust viscosity 5. Matters need attention (1)Citric acid is 50% solution. (2)Salt is 20% solution. (3)Slowly agitate. 6. Questions (1)What is the action of each component? (2)Why must control pH value? Experiment 18 Preparation of Hair-Protecting Agent 1. Aim of experiment (1) Understood the preparation principle of hair-protecting agent product. (2) Known the action of compositions in formulation. 2.Principle of experiment The hair-protecting agent is the result of maintaining, softening and lustering hair. The hair-protecting agent consist of cation surface active agent such as 1831, 1231, 1227, 1827, D1221 and hydrolyze collagen protein, and so on. The other components are oil phase and water phase. The oil phase consist of beeswax, Vaseline, white oil, olive oil, lanolin, its derivant, squalane, silicon oil, higher fatty acid ester and higher alcohols. The water phase consists of water, glycerine, propanediol, and so on. The emulsifier consists of triethanolamine soap, fatty alcohol sulfate, Span, Tween and AEO. The cation surface active agent can be absorbed by hair which can form monomolecular absorption film, making hair soft. The polymeric compounds have adding viscidity and thickening which can increase stability of system. The glycerine and propanediol are humectants. The white oil is stearol. The protein hydrolysate can promote hair grow. The vitamin E can promote cycle and anti-oxidation. 3. Main apparatus and chemical reagents Electric heater, Beaker and counter balance. 1631, octadecanol, mon- stearin, triethanolamine, OP, spice and glycerine. 4. Experiment procedures (1)The octadecanol, mon- stearin and water are put into 200ml beaker. (2)The triethanolamine, OP, spice and glycerine are put into the 200ml beaker through agitation. (3)The result is got when reactants cool. 5. Matters need attention (1)Tepidity when the raw materials are melting. 6. Questions (1)What is principle of protecting hair about hair-protecting agent? (2)What is the each component action? Experiment 20 Preparation of Detergents 1. Aim of experiment (1) Understood the preparation principle of detergent products. (2) Known the action of compositions in formulation. 2.Principle of experiment (1) Main quality and use The cleaning mixture can unoil and be health which can be called wash. (2) Principle a. innocuity, b. scour off oil dirty, c. no residual, d. good sparkle, e. kill bacterium in effect, f. good stability, g. suitable concentration and viscosity, h. higher alkalinity, i. adding protective agent. 3. Main apparatus and chemical reagents Beaker, counter balance, electric heater, water bath pot, cylinder. Dodecy benzene monosulfonic acid sodium, fatty alcohol polyethenoxy ether sodium sulfate, alcohol, formaldehyde, triethanolamine, essence, test paper, sulfuric acid. 4. Experiment procedures (1) Water is put into beaker which is heated to 60℃. (2) AES is put into the beaker through agitation until solve entirely. (3) Other surface active agents are put into beaker until melted entirely. (4) Fall and put essence and antiseptic into beaker. (5) Adjust pH value to 9-10.5 by sulfuric acid. (6) Adjust viscosity by salt. 5. Matters need attention (1) AES is put into the beaker through agitation slowly. (2)The soluble temperature can’t exceed 65℃. 6. Questions (1)Which phenomenon can be observed in the process of preparation of detergents? (2)Why do cleaning mixture control pH value to 9-10.5? Experiment 21 Estimation of Cleaner Ability of Detergents 1. Aim of experiment Understood the estimation application of cleaness ability of detergents. 2.Principle of experiment Degrease ability is the main target of cleaner. It can look for the best cleaner through estimation of cleaner degrease ability. The normal oil dirty is blot out the sheet glass which had weighing. Clean the sheet glass with certainly concentration cleaner, weigh up after dryness. Then calculate degrease ability through following formulary: W% = B-C ×100% B-A 3. Main apparatus and chemical reagents Apparatus of cleaner degrease ability estimation, sheet glass, counter balance, leg, nipper, pledget, capability bottle, and little beaker. 4. Experiment procedures (1) Blot oil dirty out the sheet glass. The six sheet glass are cleaned by alcohol. Weigh up after dryness. Each sheet glass is immerged into the normal oil dirty at 20℃; time is 3s.Put on the leg at 32℃ after wipe bottom oil. Weigh up after dryness about 1h. (2) Confection of hard water. Weigh up calcium chloride 0.165g and sal epsom 0.247g, diluting to 1L with water in capability bottle. The hard water concentration is 250ppm. (3) Confection of cleaner solution. Dilute to 1L with water in capability bottle after adding 10g cleaner. (4) The six sheet glass are put into legs of apparatus of cleaner degrease ability estimation carefully. The 700ml cleaner solution is put into 1000ml beaker which apparatus of cleaner degrease ability estimation is inside. Wash about 3min at 32℃.The 700ml water solution is put into the beaker after cleaner is spilled. Wash about 1min at 32℃.Take out six sheet glass. Weigh up after dryness about 24h. (5) calculate degrease ability. 5. Matters need attention Pay attention to serial number order. 6. Questions (1) Why do we avoid contacting sheet glass directly? (2) Why do we weigh up after dryness about 24h? Experiment 24 Preparation of Vanishing Cream 1. Aim of experiment (1) Understood the preparation principle of vanishing cream products. (2) Known the action of compositions in formulation. 2.Principle of experiment Vanishing cream is a kind of white cosmetic which emulsifier is soap of stearic acid. It contains fatty acid, fatty alcohol and polyols tallate in the oil phase. In this way vanishing cream can become protective tissue on the skin surface. The protective tissue can avoid moisture evaporation quickly for dying skin and dying area. Vanishing cream consist of soap of stearic acid(3.0-7.5%), stearic acid(10-20%), polyols(5-20%)and water(60-80%)generally. Stearic acid can become protective tissue on the skin surface; soften up corneal layer and save moisture. Alkali can become soap of stearic acid with stearic acid. The general alkali is caustic potash which can avoid dynamic viscosity of system largely. Polyols can become humectants which can avoid noodles phenomenon of vanishing cream improving endures frost. Mon- stearin is coemulsifier which can assure cream fine fairly and good luster. The effect of cetanol with mon- stearin is good which dosage is 1—3%.White oil can mix colors and avoid noodles phenomenon of vanishing cream. In addition, lanolin, apple-oil and propolis can add for functional components. 3. Main apparatus and chemical reagents Beaker, motor agitator, thermometer, microscope, counter balance, electric heater, water bath pot. Stearic acid, mon-stearin, cetanol, white oil, propanediol, caustic soda, essence, antiseptic, nicety pH test paper. 4. Experiment procedures (1)Quantify Stearic acid, mon-stearin, white oil, cetanol and propanediol which are put into 250ml beaker. Water and alkali are put into another 250ml beaker. Two beakers are heating to 90℃.The materials are melted. (2)The beaker of water is keep 20min to aseptic 90℃. (3)The water and alkali are put into oil phase through agitation. (4)Put into antiseptic when temperature fall to 50℃ and put into essence at 40℃. (5)Adjust pH value of the system. Oil phase Water phase Aseptic Mix Essence Antiseptic Result 5. Matters need attention (1) Caustic soda is added a little. (2) Agitation of falling can’t be quick avoiding bubble residual in the system. (3) The result is fine and no noodles. 6. Questions (1) Why water phase and oil phase are divided into two beakers firstly in the process of confection of vanishing cream? (2) What is the action of each component? Experiment 41 Confection of Poly (vinyl acetate) Emulsion Painting 1. Aim of experiment (1) Understood the point of free radical polymerization reaction. (2)Understood the point of emulsoid painting and method of preparation. 2.Principle of experiment Emulsoid painting is a kind of watercraft painting. No hazard for human body. It can be use to inner wall of building. It can be liked by user. This experiment adopts acetic acid ethylene monosomic free radical polymerization reaction to synthesis latex. Gemmas crowd each other to form sequent and dry film coating with water evaporation when latex is brushed on the object surface. 3. Main apparatus and chemical reagents Flask, agitator, thermometer, prolong, tap funnel, water bath pot, bead mill, plastic cup. vinyl acetate, vinol, op-10, deionized water, persulfric acid ammonia, baking soda, propylene glycol, titanium pigment, tributyl phosphate. 4. Experiment procedures (1) lysis. 30ml deionized water and 0.35g emulsifier are put into Flask. 2g vinol is put into the flask after agitation. The flask is heated to 90℃ and keeps 1h until vinol solve entirely. (2) 0.2g persulfric acid ammonia is solved into water, forming solution. (3) polymerization. The 17g vinyl acetate after distillation and 2ml persulfric acid ammonia solution which mass fraction is 5% are put into the flask. Start agitator and heat water bath pot. Keep temperature between 65℃ and 75℃. When back flow vanishes basically, 23g vinyl acetate and the rest persulfric acid ammonia solution will be dropwised into the flask slowly. The dropwising time is 2h. The dropwising temperature is 83℃. Promote temperature between 90℃ and 95℃ after dropwising end. Keep 30min in the temperature until back flow stop entirely. The 3ml baking soda solution which mass fraction is 5% is put into the flask when temperature is cooled to 50℃.Adjust pH value between 5 and 6. 3.4g phthalic acid two fourth ester is put into the flask. Cool 1h with agitation. The white thick result is get. 5. Matters need attention (1) Vinyl acetate must solve entirely. (2) Dropwising speed must equality. (3)Raising temperature can’t be too quick. (4) Vinyl acetate can be used after distillation. 6. Questions (1)What is the action of Vinyl acetate in the reaction? (2) What is the action of persulfric acid ammonia in the reaction? (3) What is the action of adjusting pH value in the reaction? Experiment 44 Synthesis and Application of Bisphenol Epoxy Resin 1. Aim of experiment (1) Understood the synthesis principle and basic operation of bisphenol epoxy resin. (2) Understood the synthesis principle and application of common epoxy resin. 2.Principle of experiment The method of two step alkalify is adopted at present which can avoid epoxy chloropropane hydrolyze high limit. So the reclamation of epoxy chloropropane hydrolyze is increased. C H3 HO OH + NaCl Cl O C H3 C H3 HO C H3 HO OH C H3 O + O NaCl C H3 C H3 HO C H3 Through: C H3 O OH O OH C H3 CH3 C H3 O O O n O OH O O CH3 C H3 3. Main apparatus and chemical reagents Flask, agitator, thermometer, prolong, tap funnel, water bath pot, bead mill, plastic cup. Bisphenol epoxy resin, sodium hydrate, benzene, spirit of salt, acetone, phenolphthalein, sheet glass, plastic piece. 4. Experiment procedures (1) Synthetic resin. 22.8g bisphenol epoxy resin and 28g epoxy chloropropane are put into flask. Melt it within 30min under 70℃. 22ml sodium hydrate solution is put into separating funnel according to the speed of 14 drop per min within 30min under 70℃. The result is refulgent 2h until the system’s color is low white yellow. (2) Lavation. Grossly lavation is done by 30ml water and 60ml benzene with agitation. The 50ml water which temperature is between 60℃ and 70℃ is put into separating funnel and wash the result. Divide the layer of water. (3)Take off benzene, water and not reactive monomer. The impurity will be evaporated out from the reactive system under 80℃-90℃.The stick resin will be leaved in the flask. (4) Estimation of epoxide number. Two samples and one blank are titrated. Weigh up 0.3-0.5g sample. 15ml spirit of salt- acetone solution, sample and 0.1% phenolphthalein indicator are put into conical flask. The 0.1mol.L-1 standard NaOH solution is titrated into the conical flask until color of the conical flask is red. Do parallel test and blank test as comparison. 5. Matters need attention (1) Dropwising speed of NaOH must be slow. 6. Questions (1)Which catalyzer is used in the reaction? What is the action of dropwising speed about catalyzer? (2) Why must do fractionation after epoxy resin synthesis? (3) How is the temperature of fractionation in the reaction? Experiment 46 Preparation of Barium Titanate (BaTiO3) Nanometer Powders Experimental purpose: 1. Familiar the sol-gel technique and master the preparative technique of BaTiO3 nanometer powder. 2. Understand the characterization method by X-ray diffraction for inorganic compounds. Experimental Principle: BaTiO3 has been applied in ceramic capacitor, multilayer film capacitor, ferroelectric storage owing to its piezoelectric and ferroelectric effect as one of important electronic materials. BaTiO3 shows tetragonal structure at room temperature, whereas it translates cubic structure at 120 oC, as shown in Fig. 1. 120℃ Fig. 1 The crystal structure of the BaTiO3 BaTiO3 was almost prepared by the equal molar ratio of the initial mixture of BaCO3 and TiO2 using solid-solid agglomeration method at 1300 oC. The reaction equation is as follow: BaCO3 + TiO2 BaTiO3 + CO2 The merit of the method is simple and cheap. However, there are a lot of disadvantage, for example high reaction temperature, non-uniformity, depending on mix muller and so on. For overcoming these disadvantages, BaTiO3 was prepared into nanometer powders using sol-gel method. The sol-gel method adopt organometallic compounds as raw materials via chemical reactions of hydrolyzation and polymerization in solution, obtained nanometer powders by sol—gel—dryness--heat treatment processes. The other method of preparation of nanometer powder is as follows: Chemical Vapor Deposition(CVD) Gas method Laser Chemical Vapor Deposition(LCVD) Vacuum Vapor and electron beam Sol-Gel liquid phase method Hydrothermal synthesis co-precipitation What are the nanometer powders? The size of the powder is in the range of 1 ~ 100 nm. Its physical character is different from macroscopical materials. For example: the piezoelectric effect of nanometer powder of BaTiO3 is more than normal BaTiO3. ~0.1 nm microcosmic 1 nm μm 100 nm nanometer macroscopical The shape and size of nanometer powder can be directly observed by electron microscope. Several nanometer particles are shown in Fig. 2. Fig. 2 The TEM images of nanometer particles Main Apparatus and Chemical Reagents: Beaker,thermometer,ground concial flask,moisturemeter,electric mantle,Al2O3 crucible,tube furnace,dryer,vacuum pump,mould. Titanium butoxide, n-butanol, anhydrous barium acetate, icy acetic acid. Experimental process: Titanium butoxide is added in butanol solution Acetic acid Titanium acylate Barium acetate hydrotropic solution transparent sol adjusting pH = 3.5 using Acetic acid gel Dried at 100 oC in air Amorphous gel dried Calcined at 800 oC Nanometer powder 20 40 60 80 100 2 Theta Characterization: D = 0.9λ/βcosθ (Scherer equation) D:mean particle diameter λ:incident X-ray wavelength (Cu target: 0.1542 nm) θ:Bragg angle(o) β:half-high width of diffraction peak at θ angle(radian) Attentions: 1. The excessive titanium butoxide is absorbed using filter paper in the course of weighing. 2. If excessive titanium butoxide have been added in butanol solution, barium acetate are also added via calculating so as to equal molar reaction of titanium butoxide and barium acetate. 3. The empty beaker was washed using 2-3 mL acetic acid, after Barium acetate hydrotropic solution has been dropped in the mixture of titanium butoxide and butanol solution. Questions: (1)Which factors should be considered while weighting Titanium butoxide? If the mass weighted overweight the theoretical mass and already added into n-butanol solution, what should we do in the next step? (2)How to ensure the weighted Ba(Ac)2 have be transferred into the n-butanol solution thoroughly? (3)One of the improved steps in present experiment is the effect prohibition of hydrolysis quickly of titanium butoxide with H2O exist in sol system. Please consider the reason, give your opinion. Experiment 84 Synthesis of Butyl Acetate 1. Aim of experiment (1) Understood the basic synthesis principle and basic operation of organic acid ester under acid catalysis. (2)Master the basic operation of back flow dividing water, lavation, dryness and distillation. 2.Principle of experiment The main reaction: O O + H3C H3C OH H3C OH O CH3 The side eaction: 13 H3C 11 12 14 4 OH 10 H3C 5 2 3 6 O 1 8 7 CH3 9 3. Main apparatus and chemical reagents Distillation flask, bulb condensation pipe, distillation conk, frank prolong, tail pipe, three-mouth flask. N- butyl alcohol, ice vinegar, concentrated sulfuric acid, soda solution, anhydr-sal epsom, pH test paper. 4. Experiment procedures (1) Back flow dividing water. 14.4mL acetic acid and 28.9mL alcohol are put into the 250mL distillation flask. The 10 drop concentrated sulfuric acid is put into the 250mL distillation flask. Several zeolites are put into. Fit the bulb condensation pipe and dividing water organ on the 250mL distillation flask. Put through condensate water. Heat distillation flask until the liquid inside it refluxes about 40min. (2) Divide the liquid and clean it. Cool when back flow end. Take the bulb condensation pipe. The layer of ester from dividing water organ and reaction liquid from three-mouth flask are put into separating funnel together. Clean with 20ml water. Divide the layer of water. (3) Remove acetic acid. Drip 25ml soda solution slowly into three-mouth flask which can be gotten together distillation cut. The gas of CO2 is transgressed after rocking the three-mouth flask continually. Check up the upper layer inside three-mouth flask with litmus paper. Divide the layer of water when the upper layer isn’t red. (4)Dryness. The layer of ester is put into dry conical flask. The anhydr-sal Epsom is put into it. Desiccate it. (5) Distillation and purification. The Butyl Acetate after dryness is shift to a dry 50mL distillation flask. Fix distillation equipment with dry instrument. Several zeolites are put into. Distill and get together the distillation cut between 124℃ and 126℃. Record the boiling point of distillate in detail. Quantify the result and calculate rate of production. 5. Matters need attention (1) Tail water when the water refluxes into the reactive system. (2) When the reaction go on about 40min and no sweat sink in the dividing water organ, the reaction end. 6. Questions (1) How do we get rid of water in the reaction? (2) How to calculate the dividing water which can ensure reaction safe. Experiment 85 Synthesis of Acetyl Cellulose 1. Aim of experiment (1) Understood the basic synthesis principle and basic operation of acetyl cellulose. (2) Understood the structure, quality and application of dimension. 2.Principle of experiment The synthesis method of plioform is that the cotton and acetic oxide go on acylation reaction in the experiment. OH OOCCH 3 O O O OH O n + ÁòËá O O O O ¼ÓÈÈ OH O C H 3 COO n OOCCH 3 If the hydroxide radical on the plioform go on acetylating, hydrogen bond between big molecules will decrease. According to acidylated degree, the plioform can dissolve into acetone and other organic solvent. Expand the application of plioform. Application: (1)Stock of synthetic drug coating. (2) Filter tip and fabric. (3) Spin fiber and film stuff. 3. Main apparatus and chemical reagents Beaker, filter flask, buchner funnel, glass water pump, water bath pot. Pledget, ice vinegar, acetic oxide, concentrated sulfuric acid, acetone, benzene, and methanol. 4. Experiment procedures (1) Acetylating of dimension. The 10g pledget, 70ml ice vinegar, 0.3ml concentrated sulfuric acid and 50ml acetic oxide are put into 400ml beaker. Cover a watch glass on the beaker and heat in water bath at 50℃. Agitate with glass rod every other cycle. Make dimension acetylating. The dimension will become homogeneous phase mash after 1.5~2h. (2) Dividing cellulose triacetate. Take half of mash and put it into another 400ml beaker. Heat to 60℃ and put 25 ml acetic acid (which mass fraction is 80%) into the beaker slowly with agitation. It can destroy excessive tri-acetic oxide. Keep 15min at 60℃. Put 25ml distilled water into the beaker. Sequentially Put 200ml water into the beaker. Cellulose triacetate will deposit from solution. Strain and clean. (3) Synthesis of 2,5-cellulose acetate. Take another half of mash and put it into another 400ml beaker. Heat to 60℃ and put 50 ml acetic acid(which mass fraction is 70%) and 0.14ml concentrated sulfuric acid into the beaker slowly with agitation. Heat 2h at 80℃ in the water bath pot. It makes cellulose triacetate partial saponification .Get the result of 2,5-cellulose acetate. Strain and clean. 5. Matters need attention (1) Concentrated sulfuric acid can’t drip into the cotton directly. (2) Concentrated sulfuric acid can drip until ice vinegar and acetic oxide soak cotton. 6. Questions (1)What is the action of concentrated sulfuric acid? Why should we pay much attention to dripping concentrated sulfuric acid during reaction? (2)Why use ice vinegar in the process of synthetic cellulose triacetate? Can we use 80% or 70% ice vinegar substitute for it? (3)Which position can occur substitute reaction in the acetylating reaction? (4)How much cellulose acetate can 5g raw materials bring theoretically? Synthesis of benzyl alcohol and benzoic acid 1. Experimental Purposes (1) To have a good command of the method and principle of Cannizarro´s reaction (2) To know other preparative methods of benzyl alcohol and benzoic acid (3) To know chief uses of benzyl alcohol and benzoic acid (4) To master separate-pure method and pure mensuration for organic solid and liquid compounds 2. Experimental Principles ——aldehyde without α-H benzyl alcohol: perfume benzoic acid: preservative The physical and chemical characters of the chief reagents apellation benzaldeh yde benzyl alcohol benzoic acid Molecular weight modality 106.13 Achromaticity liquid 108.15 Achromaticity liquid 122.13 White crystal specific gravity 1.0415 1.0419 1.2659 melting point -26 -15.3 122.4 boiling point 178.1 205.35 249 refractive index dissolvability 1.5463 Slightly dissolve in water, dissolve in ethanol, aether, acetone 1.5396 dissolve in water, ethanol, aether, acetone 1.504 Slightly dissolve in water, dissolve in hot water, ethanol, aether, acetone 3. Main apparatus and chemical reagents Beaker, flask, separating funnel, distillation flask, condensator, thermometer, tap funnel, water bath pot, balance, refraction analyzer, benzaldehyde,acther 4. Experimental Process (Erlenmeyer flask) KOH (18g, 0.32mol), water (18mL) cool to room temperature, add newly braised benzaldehyde(21g,20mL,0.2mol) placed add water (60~70mL) (separating funnel) acther layer (benzyl alcohol): 10mL saturation Na2SO3 10mL 10% Na2CO3 24h(eraser stopple) aether(20mL) extractions 10mL water(throw water layer away) dry with pure K2CO3 or pure MgSO4 braise acther in water bath 204~206℃ weight distil calculate collect compounds of productivity measure refractive index water layer(benzoic acid): acidification separate out suction strather calculate productivity coolling deposit and recrystal with water weight measure melting point 5. The most important: The separate-purify method and pure mensuration for solid compound ——recrystal, suction strather,measure melting point The separate-purify method and pure mensuration for liquid compound ——extraction, distil, measure refractive index 6. Questions? (1) The other synthesis method of benzyl alcohol and benzoic acid? (2) How to separate and purify the solid compound? (3)How to separate and purify the liquid compound Synthesis of antioxidant bisphenol A 1. Experimental Purposes (1) To have a good command of the Synthesis method and principle of antioxidant bisphenol A (2) To master the separate method of organic compounds (3) To know chemistry characters and chief uses of antioxidant bisphenol A 2. Experimental Principles secondary reaction (1) phenol oxidation (2) isomerization (adjacent product) or more condensation product (3) toluene alkylate (4) sulphonation (5) esterification how to increase productivity (1) excessive acetone (2) remove water exist problem (1) it is propitious to remove water in high temperature, but secondary reaction increase (2) excessive acetone is of benefit for phenol using, but emulsification take place easily ( how to break emulison?) The physical and chemical characters of the chief reagents dissolvability Molecular weight specific gravity melting boiling point point water ethanol aether phenol 94.11 1.0576 43.0 181.7 dissolve dissolve Easy dissolve acetone 58.08 0.7899 -95.4 56.2 ∞ ∞ ∞ toluene 92.15 0.8669 -95.0 110.6 Not dissolve ∞ ∞ bisphenol A 228.29 1.195 155.6 Slightly dissolve dissolve dissolve apellation 3. Main apparatus and chemical reagents beaker, separating funnel, tri-neck-bottle ,distillation flask, condensator, thermometer, tap funnel, water bath pot, balance phenol, acetone , toluene, sulfuric acid 4.Experiment processe (tri-neck-bottle)phenol (45g), toluene (90g, ?mL), sulfuric acid (79%-64 g, ?mL), (0.3g mercaptoacetic acid) drop acetone (﹤40℃) stir 2h (36~40℃) (separating funnel) wash with hot water (82℃, 3times 150mL-200mL-200mL, reserve which layer?) strather (breaker) crystal in cold water rough product measure melting point suction calculate productivity 5.The most important: (1) bisphenol A dissolve in acetone, toluene, but slightly dissolve in water (2) phenol dissolve in hot water, but not dissolve in cold water 6. Questions? (1) Which kind of reaction is the synthesis of antioxidant bisphenol A? (2) What is the secondary reaction? How to avoid? (3)Why the hot water (82℃) was used in this experiment? Synthesis of Acetylsalicylic Acid 1 Experimental Purposes (1)To have a good command of the synthesis principle and experimental method of acylating reaction (2) To know the application area of acylating reaction (3) To know the use of Acetylsalicylic Acid (4) To master the decontamination method of solid compound 2 Experimental Principles COOH + (CH 3CO) 2O OH Acylating agent: acyl halide, carboxylic acid, anhydride secondary reaction? H2SO4 80¡ æ COOH O O CH3 Application: i alkylation ii Protective group iii edulcorator The physical and chemical characters of the chief reagents molecular appellation specific Melting Boiling gravity point point modality weight solubility Tiny dissolved in white 211 Salicylic water, lyotropic in hot 138.12 crystal or 1.443 159 Sublimate acid water ,alcohol ,dieth powder -ion yl ether, acetone lyotropic in water, Acetyl Colorless 102.09 1.082 oxide -73.1 139.55 alcohol,diethyl ether, liquid benzene dissolved in hot Acetylsali- Acetylsali 180.17 cylic Acid 1.35 135 water ,alcohol, Tiny cylic Acid dissolved in acetone 3. Main apparatus and chemical reagents conical flask beaker, condensator, thermometer, tap funnel, water bath pot, balance salicylic acid, acetyl oxide, sulfuric acid, alcohol 4. Experimental Process (Conical flask) Salicylic acid (6.3g,0.045mol) Acetyl oxide (9.5 g,9ml,0.09mol),10 drips of concentrated sulfuric acid →heating in water bath(70℃,20min, ﹡shake) →cooling with aquae frigida → suction filtration → wash 2 times with aqua astricta → baking dry (crude product) → recrystallization(alcohol — water) → measure melting point ,calculating yield 5. The most important: (1) suction filtration (2) recrystallization (3) measure melting point 6. Questions? (1) How many acylating agents can be used in acylating reactions? when acetyl oxide was used as acylating agent? (2) What is the secondary reaction? How to avoid? How to operate