Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
Answers
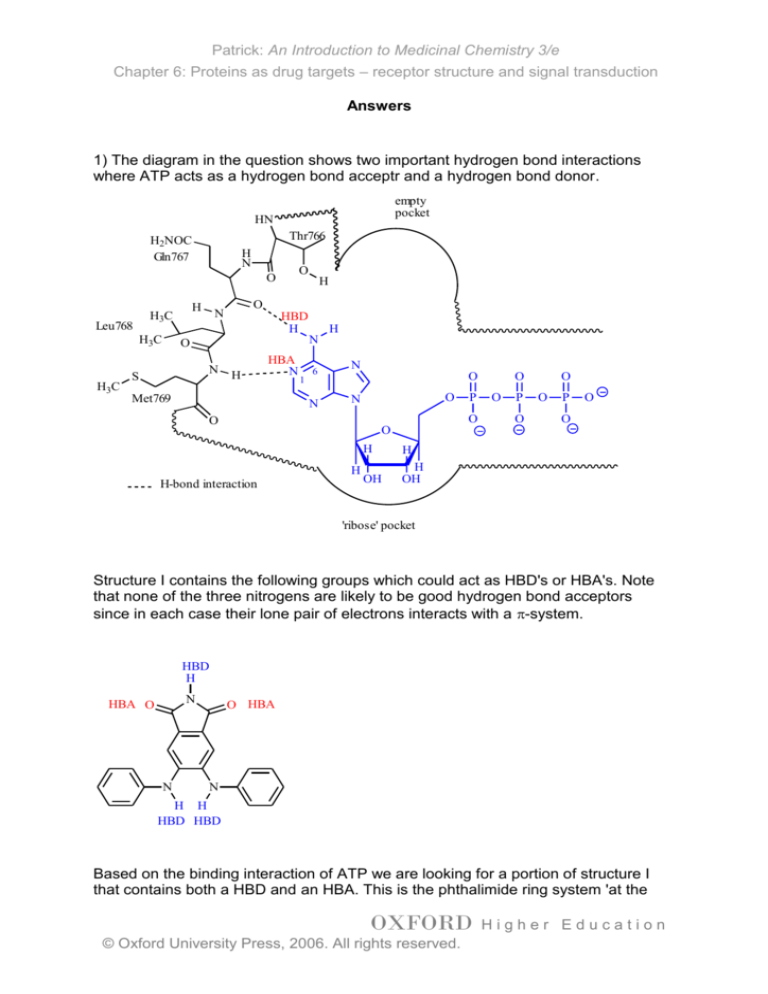
1) The diagram in the question shows two important hydrogen bond interactions
where ATP acts as a hydrogen bond acceptr and a hydrogen bond donor.
empty
pocket
HN
Thr766
H2NOC
Gln767
H
N
O
O
Leu768
H3C
H3C
H
H3C
O
N
HBD
H
O
N
S
H
HBA
N
Met769
1
H
N
6
N
H
N
O
O
N
O
O
O
H
H-bond interaction
P
H
H
OH
H
OH
O
O
P
O
O
O
P
O
O
'ribose' pocket
Structure I contains the following groups which could act as HBD's or HBA's. Note
that none of the three nitrogens are likely to be good hydrogen bond acceptors
since in each case their lone pair of electrons interacts with a -system.
HBD
H
N
HBA O
N
O HBA
N
H H
HBD HBD
Based on the binding interaction of ATP we are looking for a portion of structure I
that contains both a HBD and an HBA. This is the phthalimide ring system 'at the
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
top'. The molecule could be fitted into the binding site as shown. Note that an extra
hydrogen bonding interaction might be possible to Thr766, and that one of aniline
rings might be positioned correctly for the 'ribose' pocket. However, there is no way
one can say this for certain and molecular modelling experiments would have to be
done to see whether this is feasible.
empty
pocket
HN
Gln767
Thr766
H2NOC
H
N
O
H
Leu768
H3C
Met769
S
H
O
O
N
H
H3C
O
O
N
H
N
NH
O
H3C
HN
O
H-bond interaction
'ribose' pocket
2) In an -helix, the NH and C=O of each peptide link forms hydrogen bonds within
the centre of the helix such that these groups do not have to interact with the
hydrophobic centre of the cell membrane. The -helix also positions the side
chains (which are mostly hydrophobic) to the exterior of the helix where they can
interact with the hydrophobic centre of the cell membrane (see also 3.2).
3) It is worth considering the interactions of oestradiol and raloxifene with the
oestrogen receptor (Box 6.2) in order to answer this question.
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
His 524
Me OH
H
H
H
N
Side
chain
H
Glu353
Asp351
H
H
His 524
O
Glu353
O
OH
O
H
Hydrophic skeleton
H2O
O
S
Arg394
Oestradiol
Arg394
Raloxifene
Both oestradiol and raloxifene contain functional groups that can interact through
hydrogen bonding to the amino acids Glu353, Arg394 and His524. Both molecule
have hydrophobic skeletons that position these groups correctly and match the
hydrophobic nature of the binding site. Oestradiol is an agonist whereas raloxifene
is an antagonist. This is due to the extra interaction with Asp351 that is possible for
raloxifene.
Turning now to tamoxifen, this molecule is also hydrophobic and of a similar size to
the above, allowing it to fit the hydrophoic binding site. It does not have the phenol
or alcohol functional groups present in oestradiol or raloxifene, but it does have a
group that can interact with Asp351 in the same way as raloxifene, resulting in it
acting as an antagonist.
Asp351
H
Me2N
O
Tamoxifen
4) Although tamoxifen itself is an antagonist, its metabolite is an agonist. This is
because it has lost the group that is so crucial for antagonist activity (the side chain
containing the amine). It also contains a phenol group which can mimic the
phenolic group of oestradiol (see above question 3).
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
Asp351
Asp351
H
Me2N
HO
O
Glu353
Glu353
Tamoxifen
Arg394
H
O
Metabolite
Arg394
5) When the Ras protein is activated, it has an autocatalytic property which means
that it has a catalytic property which leads to its own deactivation without the need
for any external influence. If the autocatalytic property was lost, the Ras protein
would remain permanantly activated and this would have serious consequences on
cell chemistry since the Ras protein initiates processes such as cell growth and cell
division. Many cancers have been linked to defective Ras proteins which have lost
the ability to deactivate themselves (see also section 18.6.1).
6) Ras is synthesised in the cytoplasm of the cell and has to become attached to
the cell membrane in order to function properly. The attachment of a long
hydrophobic chain means that the alkyl group can penetrate into the hydrophobic
cell membrane and act as a hydrophobic anchor, holding the Ras protein to the
inside surface of the membrane. If the enzyme was inhibited, the anchor would not
be attached to the ras protein, and the protein would not become attached to the
membrane. as a result, it could no longer act as a signal protein and any signal
transduction processes involving the Ras protein would be interrupted (see also
section 18.6.1).
7) Signal amplification takes place at the following stages:
a) One adrenaline molecule activating a receptor results in several s subunits
being generated.
b) A single s subunit activating adenylate cyclase is responsible for generating
several cyclic AMP molecules.
c) A single cyclic AMP molecule releases the catalytic subunit of PKA which
catalyses i) the activation of several inhibitors, ii) the deactivation of several
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
glycogen synthase enzymes and c) the activation of several Phosphorylase kinase
enzymes.
d) Each activated phosphorylase kinase enzyme activates several phosphorylase b
enzymes.
e) Each phosphorylase a enzymes catalyses the breakdown of several glycogen
molecules.
Adrenaline
s
-Adrenoceptor
ATP
Glycogen
synthase
(active)
s
Adenylate
cyclase
cAMP
Protein kinase A
C
Inhibitor
(inactive)
Catalytic
subunit of
PKA
Inhibitor- P
(active)
Glycogen
synthase- P
(inactive)
Phosphorylase
kinase (inactive)
Phosphatase
(inhibited)
Phosphorylase
kinase- P (active)
Phosphorylase b
(inactive)
Phosphorylase a
(active)
Glycogen
Glucose-1-phosphate
8) An inhibitor of cAMP phosphodiesterase would prevent cyclic AMP being
hydrolysed. As a result, cyclic AMP would have a longer lifetime leading to
increased activity of PKA. This in turn would lead to increased breakdown of
glycogen
9) The natural enzyme is activated by a serine-threonine kinase which catalyses
the phosphorylation of serine and threonine side chains. Thus the side chain
alcohol of serine would be phosphorylated as shown.
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
H H
N
O
Protein
kinase A
H H
N
O
ATP
OH
Serine
O
O
P
O
O
This can have a profound effect on tertiary structure. The alcohol of serine
participates in hydrogen bonding with other hydrogen bonding groups.
Phosphorylation introduces an ionic groups which will form ionic bonds with other
ionic groups in the protein leading to a change in conformation and activation of
the enzyme. Deactivation occurs when the phosphate groups are hydrolysed to
give back the original alcohol groups. the enzyme then returns to its original
inactive conformation.
In the genetically engineered enzyme, the serine residues are replaced with
glutamate residues (-CH2CH2CO2-). The carboxylate groups are ionised and as a
result the molecule does not need to become phosphorylated to become active.
The glutamate residues form the same ionic interactions as the phosphate groups
and the enzymes is inherently active. Moreover, there is no way of removing the
charge and the the enzyme is permanantly active.
10) Tyrosine kinases catalyse the phosphorylation of the phenol group of tyrosine,
but not the alcohol groups of serine and threonine. This suggests that the active
site for a tyrosine kinase contains a hydrophobic region that can interact with the
aromatic ring of tyrosine through van der Waals interactions. Since the side chains
of serine and threonine do not contain an aromatic ring, they are not bound so
effectively, and as a result do not act as substrates.
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education
Patrick: An Introduction to Medicinal Chemistry 3/e
Chapter 6: Proteins as drug targets – receptor structure and signal transduction
H
N
H
Protein
N
N
O
O
O
H
H
O
N
N
HO
O
Tyrosine
residue
H
OH OH
Mg
Tyrosine kinas e
H
N
N
O
H
ATP
H
Protein
HN
O
O P O P O P O
N
N
O
O
N
O
O
H
H
Protein
O
H
H
OH OH
ADP
Protein
HN
O P O P O
O
O
O
O
P
O
O
Phosphorylated
ty ros ine res idue
11) The activation of growth factor receptors involves dimerisation. Antibodies are
Y-shaped molecules where the tip of each arm of the Y binds to a particular target.
As a result, an antibody can act as a bidentate ligand. Cosequently, one antibody
could bind to two different receptors, resulting in the dimerisation and activation of
the growth factor receptor.
OXFORD
© Oxford University Press, 2006. All rights reserved.
Higher Education







