Mass spectrometry- Teacher notes
advertisement

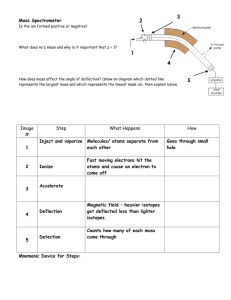
TEACHERS’ NOTES MASS SPECTROSCOPY Student learning outcomes for mass spectroscopy should include: Interpret a mass spectrum Calculate the molar mass of a compound based on the mass spectrum provided Identify the main peaks (molecular ion peak and base peak) What does mass spectrometry measure? John B. Fenn, the originator of electrospray ionization for biomolecules and the 2002 Nobel Laureate in Chemistry, probably gave the most apt answer to this question: Mass spectrometry is the art of measuring atoms and molecules to determine their molecular weight. Such mass or weight information is sometimes sufficient, frequently necessary, and always useful in determining the identity of a species. To practice this art one puts charge on the molecules of interest, i.e., the analyte, then measures how the trajectories of the resulting ions respond in vacuum to various combinations of electric and magnetic fields. Mass spectrometry is used to find out the molecular mass of a compound and it is also possible to determine the elemental composition of a substance. Advantages of mass spectroscopy are that it has high sensitivity and high accuracy. A mass spectrum is obtained by converting components of a sample into gaseous ions and separating them due to their mass-to-charge ratios. It is based on the motion of a charged particle, an ion, in an electric or magnetic field. A mass spectrum provides information about: The quality and quantity of the inorganic and organic components in a mixture The structures of a wide variety of molecular species The ratio of isotopes in a sample Structure and composition of solid surfaces Mass spectrometry enables the mass of individual ions to be determined from compounds in the gas phase. How is a mass spectrum produced? Microgram quantities of the pure compound are vaporised It is then bombarded with electrons in an ionising chamber The molecules lose 1 electron to form a positive ion, some of this breaks down to smaller ions by losing radicals or neutral molecules The positive ions are filtered out as they are accelerated through an electric field They are then analysed as they are deflected through a magnetic field Finally they are detected by an ion collector A recorder prints the spectrum as a graph of the relative abundances of their ions against their mass to charge ratio The mass to charge ratio (m/z): m is the numerical value for the mass of the ion and z is the numerical value for the charge of the ion. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. An ion with a mass of 56 and a charge of 2+ would also have a mass/charge ratio of 28. The ionised molecule occurs as one electron (usually) is expelled forming a radical cation. M → M• + + • (e-) Example of a mass spectrum of pentan-3-one: www.chemguide.co.uk The peak at 86 would be the molecular ion peak. This corresponds to the molecular weight of a singly charged molecule, CH3CH2COCH2CH3• + . CH3CH2COCH2CH3• + = 5 carbons (5x12) + 10 hydrogens (10 x 1) + 1 oxygen ( 1 x 16) = 86 The peak at 57 would be the base peak (highest peak). This is shown as the most intense m/z (highest relative abundance). This corresponds to CH3CH2CO+ . The peak at 29 would correspond to the ethyl ion CH3CH2+. Note: the more stable an ion the more likely it is to form. eg. In the case of carbocations 3°>2°>1° Why is mass spectroscopy useful? By performing mass spectroscopy on substances you could: Work out the chemical structures of molecules such as carbohydrates, nucleic acids and peptides Detect and identify the use of steroids in athletes Detect dioxins (poisons) in contaminated fish Determine the isotopes of an element within a sample 1. A useful 8 minute video can be found on Youtube. www.youtube.com/watch?v=J-wao0O0_qM This is produced by the Royal Society of Chemistry and explains how the spectrometer works and how mass spectra are produced. 2. www.chemguide.co.uk Go to Instrumental analysis, mass spectroscopy. This has good explanations of organic compounds containing halogen atoms. 3. The following website contains ‘A self-guided introduction to mass spectrometery’. Includes how a mass spectrometer works. Problems for: Interpreting mass spectra Drawing mass spectra Working out fragments shown on mass spectra www.ccmr.cornell.edu/education/modules/documents/WhatsMassGottoDoWithIt.pdf 4. The website www.rod.beavon.clara.net/spectra.htm contains a range of mass spectra and infrared spectra for selected alcohols, aldehydes, ketones, carboxylic acids, amines and an ester and acid chloride. ‘A mass spectrometrist is someone who figures out what something is by smashing it with a hammer and looking at the pieces’. www.research.uky.edu 5. The website http://www.vicsco.com.au/downloads/Mass_Spec.pdf contains brief notes on what mass spectroscopy is used for and how to read a mass spectrum