Fundamental Physics Notes
Joseph E. Johnson, PhD
Professor of Physics
University of South Carolina
July 24, 2007 Version
© Joseph E. Johnson 2006 All rights Reserved
1
Fundamental Physics
Table of Contents
Joseph E. Johnson, PhD © 2006
1. Mechanics
1.1. Newtonian Mechanics
1.1.1. Introduction
1.1.2.Kinematics in One Dimension
1.1.3.Kinematics in Two & Three Dimensions
1.1.4.Forces & Newtons Laws of Motion
1.1.5.Uniform Circular Motion
1.1.6.Work & Energy
1.1.7.Momentum and Impulse
1.2. Rotational Mechanics & Gravity
1.2.1.Rotational Kinematics
1.2.2.Rotational Dynamics
1.2.3.Gravitation
1.3. Solids, Fluids, & Waves
1.3.1.Elasticity
1.3.2.Simple Harmonic Motion
1.3.3.Fluids
1.3.4.Mechanical Waves & Sound
1.3.5. Linear Superposition of Waves, Interference, & Music
2. Thermodynamics
2.1.1.Temperature & Heat
2.1.2.Transfer of Heat
2.1.3.Ideal Gas Law & Kinetic Theory
2.1.4.Thermodynamics
3. Electromagnetic Theory
3.1. Electricity
3.1.1.Electric Forces
3.1.2.Electric Field
3.1.3. Gauss’ Law
3.1.4.Electric Potential & Potential Energy
3.1.5.Capacitance
3.1.6. Electric Current & Resistance
3.1.7.Direct Electrical Currents
3.2. Magnetism
3.2.1.Magnetic Fields
3.2.2.Magnetic Field Sources
3.2.3.Faraday’s Law
3.2.4.Induction
3.2.5.Alternating Electric Currents
3.3. Electromagnetism
3.3.1.Maxwell’s Equations
3.3.2.Solution in a Vacuum – EM Waves
4. Light & Optics
4.1.1. Reflection of Light & Mirrors
4.1.2. Refraction of Light & Lenses
4.1.3. Interference & Wave Nature of Light
5. Relativity
5.1.1. Special Relativity
5.1.2. General Relativity & Astrophysics
6. Quantum Theory – Atomic, Nuclear, & Particle Physics
6.1.1. Foundations of Quantum Mechanics – Particles & Waves
6.1.2. Atomic Theory
6.1.3. Nuclear Theory & Radioactivity
6.1.4. Elementary Particle Theory
7. Mathematics Background
8. Some Useful Numerical Value and Relationships
9. How to best process this material as a Physics Course
CJ = Cutnell & Johnson: Physics 7th Edition 2007
SV = Serway & Vuille: Essentials of College Physics 2007
<CJ - 1 & SV 1 >
<CJ - 2 & SV 2>
<CJ - 3 & SV 3>
<CJ - 4 & SV 4>
<CJ - 5 & SV 7>
<CJ - 6 & SV 5>
<CJ - 7 & SV 6>
<CJ – 8 & SV 7>
<CJ - 9 & SV 8>
<CJ - 4.7, 9.3 & SV 7>
<CJ - 10.1, 10.7, 10.8 & SV 9>
<CJ - 10 & SV 13>
<CJ - 11 & SV 9>
<CJ - 16 & SV 14>
<CJ - 17 & SV 13, 14 >
<CJ - 12 & SV 10>
<CJ - 13 & SV 11>
<CJ - 14 & SV 10>
<CJ - 15 & SV 12>
<CJ - 18.1-18.5 & SV 15 >
<CJ - 18.6-18.8 & SV 15>
<CJ - 18.9 & SV 15>
<CJ - 19.1-19.4 & SV 16>
<CJ - 19..5-19.7 & SV 16>
<CJ - 20.1-20.7 & SV 17>
<CJ - 20.8-20.15 & SV 18>
<CJ - 21.1-21.6 & SV 19>
<CJ - 21.7-21.10 & SV 19>
<CJ - 22.1-22.6 & SV 20>
<CJ - 22.7-22.10 & SV 20>
<CJ - 23 & SV 21>
<CJ - 24.1-24.3 & SV 21>
<CJ - 24.4-24.7 & SV 21>
<CJ - 25 & SV 22, 23>
<CJ - 26 & SV 23, 25>
<CJ - 27 & SV 24>
<CJ - 28 & SV 26>
<CJ - 28.8 & SV 26>
<CJ - 29 & SV 27>
<CJ - 30 & SV 28>
<CJ - 31 & SV 29>
<CJ - 32 & SV 30>
<CJ - 1 & Appendix >
ISBN 0-471-66315-8
ISBN 0-495-11129-5
2
Preface
These notes have been compiled in order to summarize the core concepts, definitions, terms,
equations, and relationships for an introductory Physics course. My objective is to provide the student with
an outline of the very essentials which are to serve as a guide to my lectures and any of the very well written
texts that are available and to keep the focus on the core ideas as it is easy for a student to become
overwhelmed or lost in the more than one thousand page texts and the massive information that is conveyed
in the lectures. These notes are the skeletal framework upon which one can attach the rest of the material.
I have separated each chapter or topic into a separate page thus allowing one to print these pages
from the web for personal use with space for taking ones own notes during lecture and later with the text in
hand. Each chapter or topic is further divided into three areas: (1) Descriptive, (2) Mathematical, and (3)
Advanced. The ‘Descriptive’ part covers the non-mathematical parts that might be covered in a course
such as ‘Physical Science’ or ‘Physics in the Arts’ that is generally devoid of algebra and trigonometry. The
‘Mathematical’ part covers introductory physics at the level of algebra and trig but without calculus. Such a
course is customary for the health and biological sciences. Such a course naturally includes the descriptive
level as well. Finally the ‘Advanced’ section of a chapter includes calculus at both the introductory and
advanced level (of vector calculus) along with differential equations and some use of linear algebra and
matrix theory along with both the descriptive and mathematical sections. I have found that almost all
students today in the biological sciences (pre-med, pre-dental, …) have had calculus and thus I use the
advanced concepts even in the non-calculus course for edification, but I do not test them at that advanced
level as I do with the physics, chemistry, geology, mathematics, and engineering students who all take the
Calculus level course.
I have used red fonts for equations and green fonts for numerical values and constants. This allows
their rapid recognition. I often use web-available software which I have developed for UNITS conversion
as an environment that allows one to mix units in any valid way thus providing an environment for very rapid
computation. Finally, I also am testing an on-line (Internet) prompt-response system for tests, quizzes,
homework, polls, and demographic data collection. Both the UNITS and Prompt-Response System are in
Beta testing during the Spring of 2007 in conjunction with my teaching of the Physics 202 (second semester)
course. I am likewise developing on-line lectures that can be used as a supplement to my regular class
lectures for additional review and for students who missed the lecture. These video lectures are designed to
capture a chapter in no more than 30 minutes as I have found that I am able to cover a one hour lecture in
that time if there are no interruptions, and no repeated material. This time is devoted to rapidly covering just
the core concepts allowing the student to replay the lecture as is necessary. A version of these lectures for
the IPOD is being made available for downloads.
I intend to modify these lecture notes on a continuous basis using the Internet site for posting. Thus I
can correct typos and make enhancements as are required to the material. I welcome comments and
suggestions (at jjohnson@sc.edu) to the general framework of these components Notes, Video Lectures,
UNITS software, and the Question-Response software all of which can be found at www.asg.sc.edu
Joseph E. Johnson, PhD
Professor of Physics
University of South Carolina
Columbia, SC, 29208
August 18, 2007
3
Fundamental Physics
Joseph E. Johnson, PhD © 2006
1. Mechanics
1.1. Newtonian Mechanics
1.1.1. Introduction <CJ chap 1 >
1.1.1.1. Discussion
1.1.1.1.1. Units
1.1.1.1.1.1. One Meter = distance that light travels in a vacuum in 1/299,792,458 s
1.1.1.1.1.1.1. Scales of distance: quark-quark, atom, virus, human, earth, to sun, galaxy, universe
1.1.1.1.1.2. One Kilogram = the mass of a platinum-iridium cylinder in Paris (mass of 1/1000 of m 3 of water)
1.1.1.1.1.2.1. Scales of masses: electron, proton, .. human, planet, star, galaxy
1.1.1.1.1.3. One Second = the time of 9,192,631,770 vibrations of Cesium 133 radiation
1.1.1.1.1.3.1. Scales of time; light across proton, cesium, lifetime of human, age of earth, universe
1.1.1.1.1.4. Discuss derived units: m/s, kg/m, m2 , m3
1.1.1.1.1.5. Correct use of units +- only of same types, */ any kinds, transcendental functions (dimensionless)
1.1.1.1.1.6. Unit conversion by forming unity with which one can * and /
1.1.1.1.2. Powers of 10 & Prefixes
1.1.1.1.3. Use of the Greek Alphabet as additional symbols
1.1.1.1.4. Numerical Uncertainty
1.1.1.1.4.1. Rules for addition and multiplication with numerical uncertainty
1.1.1.2. Mathematical
1.1.1.2.1. Vector Addition, Subtraction, & multiplication by a constant – Linear Vector Space
1.1.1.2.1.1. Graphical method
1.1.1.2.1.2. ijk method
1.1.1.2.1.3. Component form: (x, y, z) = (x1, x2, x3) = xi
1.1.1.2.2. Products
1.1.1.2.2.1. Scalar Product A * B = AxBx + AyBy + AzBz = AB cos a scalar value)
1.1.1.2.2.2. Cross Product (A x B)iijk Aj Bk = AB sin in magnitude with direction from RHR
1.1.1.2.3. The dimension of a space is the number of numbers needed to specify a point.
1.1.1.3. Advanced
1.1.1.3.1. Vectors Addition, Subtraction, & multiplication by a constant – Linear Vector Space
1.1.1.3.1.1. Ordered n-tupe Method
1.1.1.3.1.2. Scalar Product AB cos Metric Space
1.1.1.3.1.3. Cross Product AB sin - ijk symbol use
4
1.1.2.Kinematics in One Dimension <CJ chap 2 >
1.1.2.1. Discussion
1.1.2.1.1. Methodology:
1.1.2.1.1.1. A single mass moves in three dimensions of space over time
1.1.2.1.1.2. Motion in three dimensions can be understood as three independent one dimensional motions
1.1.2.1.1.3. The internal behavior of the single mass can be ignored – its position is at the center of mass
1.1.2.1.1.4. The ‘state of a particle’ is given by the position and velocity at one instant of time in its motion
1.1.2.1.1.4.1. Velocity is defined as v = x / t with units of m/s
1.1.2.1.1.4.2. Acceleration is defined as a = v / t with units of m/s2
1.1.2.1.1.4.3. Graphical view of v and a
1.1.2.1.1.5. We seek to predict its motion: given position and velocity at one time, find them in the future
1.1.2.1.2.
Simple problems:
1.1.2.1.2.1. When velocity is constant
1.1.2.1.2.2. When acceleration is constant
1.1.2.2. Mathematical
1.1.2.2.1.1. A single mass moves in three dimensions of space over time x(t)
1.1.2.2.1.2. We seek to predict its motion: given x(0) and v(0) then what is x(t) and v(t)
1.1.2.2.1.3. Define average velocity v = (x(t) – x(0) ) / t
1.1.2.2.1.4. Define average acceleration a = (v(t) –v(0))/ t
1.1.2.2.2. Simple problems:
1.1.2.2.2.1. When velocity is constant v(t) = v(0) and x(t) = x(0) + v(0) t
1.1.2.2.2.2. When acceleration is constant v(t) = v(0) + at and x(t) = x(0) + v(0) t + ½ a t2
1.1.2.2.2.3. Another equation is obtained on eliminating time: v(t)2 – v(0)2 = 2 a d where d = x(t) –x(0)
1.1.2.2.3. Constant gravity problems
1.1.2.2.3.1. a = g = 9.8 m/s2 or = 32 f/s2
1.1.2.2.3.2. v(t) = 0 at top of motion
1.1.2.2.3.3. a(t) = a = g all the time
1.1.2.2.3.4. v(0) = v(t) when the object rises and then falls back to the same height that it originally had
1.1.2.2.4. Terminal velocity – of a human 140 mi/hr max drag (spread) and 240 mi/hr minimum drag (standing)
1.1.2.2.5.
1.1.2.3. Advanced
1.1.2.3.1.1. Define instantaneous velocity v = dx(t) / dt
1.1.2.3.1.2. Define instantaneous acceleration a = dv(t) / dt
1.1.2.3.2.
Simple problems – derive:
1.1.2.3.2.1. When velocity is constant v(t) = v(0) and x(t) = x(0) + v t
1.1.2.3.2.2. When acceleration is constant v(t) = v(0) + at and x(t) = x(0) + v(0) t + ½ a t 2
1.1.2.3.2.3. Another equation is obtained on eliminating time: v(t) 2 – v(0)2 = 2 a d where d = x(t) –x(0)
5
1.1.3.Kinematics in Two & Three Dimensions <CJ chap 3 >
1.1.3.1. Discussion
1.1.3.1.1. Graphical view of projectile motion in two dimensions
1.1.3.1.1.1. Vertical motion is as in one dimension with constant a = g
1.1.3.1.1.2. Horizontal motion is as though a =0 and v = constant
1.1.3.1.1.3. Compare to view of one dimensional motion from a moving car or train
1.1.3.1.2. Graphical view of motion in a river or with an air current using vectors graphically
1.1.3.2. Mathematical
1.1.3.2.1. Projectile motion using vectors r(t) = (x(t) , y(t) ) and v(t) = (vx(t) , vy(t))
1.1.3.2.1.1. Vertical motion is as in one dimension with constant a = g
1.1.3.2.1.2. Horizontal motion is as though a =0 and had v = constant
1.1.3.2.1.3. Combined motion of vertical & horizontal
1.1.3.2.2. Graphical view of motion in a river or with an air current using vectors graphically
1.1.3.2.2.1. Compound motion by adding vectors of person relative to water and water to ground.
1.1.3.2.2.2. Determine angle of real motion, angle necessary to stay still, time across water etc
1.1.3.2.2.3. Combined velocity of airplane & wind velocity
1.1.3.3. Advanced
1.1.3.3.1. Derivation of constant acceleration equations using dv/dt = a (constant)
1.1.3.3.2. More complex projectile problems
1.1.3.3.2.1. Projectile which goes over a cliff
1.1.3.3.2.2. Projectile in moving air
6
1.1.4.Forces & Newton’s Laws of Motion <CJ chap 4 >
1.1.4.1. Discussion
1.1.4.1.1. Mass as a measure of inertia, the resistance to acceleration. - units of kg
1.1.4.1.2. Forces are vectors
1.1.4.1.3. Inertial reference frame
1.1.4.1.4. Newton’s Laws: Force measured in Newtons Nt = kg m/s2
1.1.4.1.4.1. First Law: F=0 implies a =0 and conversely
1.1.4.1.4.2. Second Law: F= ma
1.1.4.1.4.3. Third law F1->2 = - F2->1
1.1.4.1.5. Fundamental forces:
1.1.4.1.5.1. Gravitational (all masses and energy – infinite range) 10-39
1.1.4.1.5.2. Weak (involving leptons and neutrinos, very short range) 10-14
1.1.4.1.5.3. Electromagnetic (involving charged particles and currents – infinite range) 10-2
1.1.4.1.5.4. Nuclear (range of 10-15 m: p & n bound by pions) 1
1.1.4.1.5.5. Strong (quarks bound by gluons) 10
1.1.4.1.6. Frictional Force (static & dynamic)
1.1.4.1.7. Centripetal Force (from circular motion with only a change in direction)
1.1.4.1.8. Elastic force (system near equilibrium as with a spring) – Hooke’s law
1.1.4.1.9. Force of tension
1.1.4.2. Mathematical
1.1.4.2.1. Newton’s Laws
1.1.4.2.1.1. First Law: F=0 implies a =0 and conversely
1.1.4.2.1.2. Second Law: F= ma (for constant mass situations)
1.1.4.2.1.2.1. More accurately F= p/t
1.1.4.2.1.3. Third law F1->2 = - F2->1
1.1.4.2.2. Forces
1.1.4.2.2.1. Gravitational Force Fgrav = G m1 m2 / r2 and Near the earth’s surface Fgrav = W = mg
1.1.4.2.2.2. Electrical & Magnetic Force Fem = q E + q v x B where F = k q1 q2 / r2
1.1.4.2.2.3. Frictional Force (static & dynamic) Ffric = Fnormal
1.1.4.2.2.4. Elastic Force near equilibrium Felas = -kx where x is the distance from equilibrium
1.1.4.2.2.5. Centripetal force Fcen = m v2 /r where r is the radius of curvature
1.1.4.2.2.6. Force of tension is equal to the force with which the rope is pulling.
1.1.4.2.2.7. Equilibrium as Ftotal = 0
1.1.4.2.3. Resolution of forces & their vector nature
1.1.4.2.3.1. Atwood’s Machine
1.1.4.2.3.1.1. Force of tension
1.1.4.2.3.2. Incline plane
1.1.4.2.3.2.1. Without friction – one mass
1.1.4.2.3.2.2. With friction – one mass
1.1.4.2.3.2.3. With friction and two masses - tension
1.1.4.2.3.3. Problems with vector force resolution
1.1.4.2.3.3.1. Problem with rope stretched horizontally with weight
1.1.4.3. Advanced
1.1.4.3.1. Newton’s second law: F = dp/dt = d(mv)/dt or when m= const, F=mdv/dt =ma
1.1.4.3.1.1. Thus for each direction: Fx = dpx / dt , etc.
7
1.1.5.Uniform Circular Motion <CJ chap 5 >
1.1.5.1. Discussion
1.1.5.1.1. Definition of uniform circular motion with velocity v and radius r
1.1.5.1.2. Centripetal (means directed toward a center) acceleration
1.1.5.2. Mathematical
1.1.5.2.1. Period T of circular motion is defined by v = 2r / T
1.1.5.2.2. acen = v2 / r thus Fcen = m acen
1.1.5.2.3. Problem of balancing friction with centripetal forces of a car driving around a curve– flat road
1.1.5.2.4. Same problem of car on a curve but with a road that is angled
1.1.5.2.5. Problem of satellites in circular orbit GmM/r2 = m v2/r thus v = (GM/r)1/2
1.1.5.2.6. Artificial gravity using circular motion
1.1.5.2.7. Problem of pail of water rotated in a vertical plane
1.1.5.3. Advanced
8
1.1.6.Work & Energy <CJ chap 6 >
1.1.6.1. Discussion
1.1.6.1.1. Work requires energy and are often considered synonymous –
1.1.6.1.2. Energy is conveyed from one system to another exactly by the work done.
1.1.6.1.2.1. More precisely, an increase in energy is always equal to (and due to) work that is done
1.1.6.1.3. Work is defined as the force times the distance moved in the direction of work – push a lawn mover
1.1.6.1.4. The unit of work is the Joule (J) = 1 Nt acting through 1 m i.e. 1J = 1Nt*1m
1.1.6.1.5. Work and energy are scalar quantities with no direction and are not vectors.
1.1.6.1.6. Types of energy:
1.1.6.1.6.1. Kinetic – energy of motion
1.1.6.1.6.2. Potential – energy due to position or configuration
1.1.6.1.6.3. Chemical – stored for possible energy releasing chemical reactions of atoms and molecules
1.1.6.1.6.4. Nuclear – stored for possible energy releasing nuclear reactions
1.1.6.1.6.5. Solar & radiant – energy from light and more generally electromagnetic radiation
1.1.6.1.6.6. Heat – energy due to the random motion of molecules and constituents
1.1.6.1.7. Power is defined as the rate of doing work or expending energy
1.1.6.1.7.1. Energy is often defined in terms of power times time, e.g. KWHR = 1000 J/s *3600 s
1.1.6.1.8. Conservative and nonconservative forces – path independence of work & reversible
1.1.6.2. Mathematical
1.1.6.2.1. W = F r = F r cos
1.1.6.2.2. Kinetic Energy KE = W = F r = m (v/dt) r = m v v thus calculus will lead to: KE = ½ mv2
1.1.6.2.3. Gravitational Potential Energy W = Fgrav r = m g h or PE = mgh
1.1.6.2.4. Elastic Potential Energy W = Felas r = kx x thus calculus will lead to PE = ½ kx2
1.1.6.2.5.
1.1.6.3. Advanced
1.1.6.3.1.
W=
F dr and is conservative if this integral is path independent (or zero for any closed curve)
1.1.6.3.2.
1.1.6.3.3.
1.1.6.3.4.
Kinetic Energy KE = dW = F dr = m (dv/dt) dr = m v dv thus KE = ½ mv2
Gravitational Potential Energy dW = Fgrav dr = m g dh or PE = mgh
Elastic Potential Energy dW = Felas dr = kx dx thus PE = ½ kx2
9
1.1.7.Momentum and Impulse <CJ chap 7 >
1.1.7.1. Discussion
1.1.7.1.1. Impulse is defined as the change in momentum of an object such as a baseball when hit
1.1.7.1.2. Thus Impulse is a vector quantity and is often useful when the force is a complicated function of time
1.1.7.1.3. Momentum is conserved in a system that has no outside forces acting upon it.
1.1.7.2. Mathematical
1.1.7.2.1. Momentum p = m v
1.1.7.2.1.1. For any system of particles with momentum pi one has
1.1.7.2.1.1.1. P /t= pi) /t= ji Fj on i + i Fexti = 0 + Fext total because Fj on i = - Fi on j
1.1.7.2.1.1.2. Thus if there is no total external force on a system, the internal forces cancel
1.1.7.2.1.1.3.
and thus the total internal momentum is conserved.
1.1.7.2.2. Impulse = p = <F> t = the average force times the time interval.
1.1.7.2.2.1. Problem of hit baseball, & of rain verses hail on car roof (twice the impulse due to recoil)
1.1.7.2.3. Elastic collisions: Total kinetic energy after collision is same as before collision
1.1.7.2.3.1. Problem: 1 dimension – must use cons. of both energy & momentum to compute v1 & v2 after
1.1.7.2.3.2. Example of superball – bounce is essentially to equal to the previous height
1.1.7.2.4. Partially Inelastic collisions: Some kinetic energy is lost to heat from the objects collisions
1.1.7.2.4.1. Example of a bouncing ball – loss of KE is exactly measured by mgh via loss in height
1.1.7.2.5. Totally inelastic collisions: Objects stick together after collision & the maximum possible loss of KE
1.1.7.2.5.1. When object stick together there is only one v after collision which is obtained by cons. of mom.
1.1.7.2.5.2. Ballistic pendulum (bullet into a block of wood – velocity is obtained by height)
1.1.7.2.5.3. Two football players where one tackles the other
1.1.7.2.6. Center of Mass R = i mi ri / M where M = i mi = total mass of the system
1.1.7.2.6.1. Recall from above that P /t= pi) /t= ji Fj on i + i Fexti = 0 + Fext total
1.1.7.2.6.2. Thus P /t= miri / t) /t = Fext total = (MV)/ t = M V where V = velocity of COM
1.1.7.2.6.3. It also follows that P = M V
1.1.7.3. Advanced
1.1.7.3.1. Momentum p = m v
1.1.7.3.1.1. For any system of particles with momentum pi one has
1.1.7.3.1.1.1. dP /dt= d pi) /dt= ji Fj on i + i Fexti = 0 + Fext total because Fj on i = - Fi on j
1.1.7.3.1.1.2. Thus if there is no total external force on a system, the internal forces cancel
1.1.7.3.1.1.3.
and thus the total internal momentum is conserved.
1.1.7.3.2. Elastic collisions: Kinetic energy after collision is same as before collision
1.1.7.3.2.1. Problem: 2 dimensional – must use cons. of both energy & momentum to compute v1 & v2 after
1.1.7.3.2.2. Example of billiard balls
1.1.7.3.3. Center of Mass R = i mi ri / M where M = i mi = total mass of the system
1.1.7.3.3.1. Recall from above that dP /dt= d pi) /dt= ji Fj on i + i Fexti = 0 + Fext total
1.1.7.3.3.2. Thus dP /dt= dmidri /dt) /dt = Fext total = d (MV)/dt where V = dR/dt =velocity of COM
1.1.7.3.3.3. It also follows that P = M V
10
1.2. Rotational Mechanics & Gravity
1.2.1.Rotational Kinematics <CJ chap 8 >
1.2.1.1. Discussion
1.2.1.1.1. Definition of angle in radians = s / r where s is the arc length subtended & r is the radius
1.2.1.1.1.1. Thus cycle= 2 r / r = 2 radians = 360 degrees when considering the arc of an entire circle.
1.2.1.1.1.2. Circular motion restricts the distance to be a constant value of r from a given point
1.2.1.2. Mathematical
1.2.1.2.1. Define angular velocity = / t in units of radians per second or rad/s
1.2.1.2.2. Define angular acceleration = / t in units of radians per second squared or rad/s2
1.2.1.2.3. Since s = rit follows that s/t = v = r and v/t = a = r
1.2.1.2.4. If is constant then it follows that t in analogy with v = v0 + a t for translational motion
1.2.1.2.5. Likewise it follows that t + ½ t2 in analogy with x = x0 + v0t + ½ a t2
1.2.1.2.6. Combining these equations by eliminating t we obtain
1.2.1.2.7. Centripetal acceleration acen = v2/r = r 2
1.2.1.2.8. Rolling motion problems: the tangential velocity is equal to the velocity of the center of the circle
1.2.1.3. Advanced
1.2.1.3.1. Define angular velocity = d / dt in units of radians per second or rad/s
1.2.1.3.2. Define angular acceleration = d / dt in units of radians per second squared or rad/s 2
1.2.1.3.3. Since s = rit follows that ds/dt = vtan = r and dv/dt = atan = r
1.2.1.3.4. If is constant then d = dt thus t in analogy with v = v0 + a t for translational motion
1.2.1.3.5. Then using d/dt = we get t + ½ t2 in analogy with x = x0 + v0t + ½ a t2
1.2.1.3.6. Combining these equations by eliminating t we obtain
1.2.1.3.7. Vector nature of circular motion uses the RHR to get the directions of , and
11
1.2.2.Rotational Dynamics <CJ chap 9 >
1.2.2.1. Discussion
1.2.2.1.1. Just as forces give acceleration in translational motion, torques give angular acceleration in rotation
1.2.2.1.1.1. Thus Torque is to rotations as Force is to translations
1.2.2.1.2. For solid objects and systems, we can generally express the motion in translation & rotation
1.2.2.1.2.1. The translation is of the center of mass while the rotation is about the center of mass or an axis
1.2.2.1.3. Just as translational equilibrium has a net force of zero, rotational equilibrium means no torque
1.2.2.1.3.1. So equilibrium problems can be solved by requiring that the total torque (and force) is zero
1.2.2.2. Mathematical
1.2.2.2.1. Torque defined
1.2.2.2.1.1. Imagine a system with one fixed point (an axis or center) and a force is applied a distance r away
1.2.2.2.1.2. Torque is defined as the distance to the force application point times the normal force, F sin
1.2.2.2.1.3. Thus torque is defined as r x F with the right hand rule governing the direction of
1.2.2.2.1.4. Units of torque are Newtons x meters = Nm
1.2.2.2.1.5. Equilibrium is defined by i= 0 and Fi= 0
1.2.2.2.1.6. Problem: Opening a door
1.2.2.2.1.7. Problem: Using a lug wrench or screw driver
1.2.2.2.1.8. Problem: Force to support the end of a bridge – sum of several torques
1.2.2.2.2. Center of Gravity = Center of mass with weights replacing masses after multiplication by g –prove:
1.2.2.2.2.1. How to find the center of gravity of an object - hanging it from two points (intersection of verticals)
1.2.2.2.3. Moment of Inertia defined by I = i miri2 with units of kg m 2
1.2.2.2.3.1. r x F = r Fnor = r ma (but a = r) thus = m r2 which holds for each particle in a system
1.2.2.2.3.2. Thus for an ensemble of particles = (imi ri2 ) = I
1.2.2.2.3.3. Problem: Moment of inertia for different objects
1.2.2.2.3.3.1. Solid Sphere I=2/5 MR2 ; Hollow Sphere I=2/3 MR2 ; Solid Cylinder I=1/2 MR2
1.2.2.2.3.3.2. Rod with axis perp to center I=1/12 ML2 ; Rod with axis perp to end I=1/3 ML2
1.2.2.2.3.4. Problem: Object rolling down a hill
1.2.2.2.4. Rotational Work (Energy) W = F s =(Fnor r) = thus W=
1.2.2.2.4.1. Rotational Kinetic Energy KE = ½ m v2 = ½ m v2 = ½ m r2 2 thus KE = ½ I2 (units = Joules)
1.2.2.2.4.2. Problem: energy of rotating object
1.2.2.2.4.3. Problem: total kinetic energy KE = ½ m v2 + ½ I2
1.2.2.2.5. Angular momentum: = r Fnor = r p/t = r mv/t = r mr /t = (I)/t
1.2.2.2.5.1. Define angular momentum = L = Ithen = L/t and compare to F=p/t
1.2.2.3. Advanced
1.2.2.3.1. Torque is defined r x F with the right hand rule in units of Nm
1.2.2.3.2. Moment of Inertia defined by I = i miri2 with units of kg m 2
1.2.2.3.2.1. r x F = r Fnor = r ma (but a = r) thus = m r2 which holds for each particle in a system
1.2.2.3.2.2. Thus for an ensemble of particles = (imi ri2 ) thus = I
1.2.2.3.3.
Rotational Work (Energy) W =
F ds =
(Fnor r) =
d thus W=
1.2.2.3.4. Angular momentum: = r Fnor = r dp/dt = r mdv/dt = r mr d/dt = d (I)/dt
1.2.2.3.4.1. Define angular momentum = L = Ithen = dL/dt and compare to F=dp/dt
12
1.2.3.Gravitation <CJ chap 4.7, 9.3 >
1.2.3.1. Discussion
1.2.3.1.1. Newton’s law of gravitation: Every mass attracts every other mass with a force along lines of centers
1.2.3.1.2. Cavendish (1731-1810) was the first to measure the constant G giving the strength of the force
1.2.3.1.2.1. G = 6.673E-11 Nm2/kg2
1.2.3.1.3. In 1916 Einstein’s general theory of gravitation showed that even energy (eg light) is also attractive
1.2.3.1.3.1. Furthermore gravity was shown to be a curvature of space and time that altered mass motion
1.2.3.1.3.2. With black holes, this curvature is so severe that not even light can escape the attraction
1.2.3.2. Mathematical
1.2.3.2.1. Newton’s Law of Gravitation: F1->2 = - G m1 m2 / |r2-r1|2 directed as an attraction along lines of centers
1.2.3.2.2. Gravity near the surface of a planet: F = m g where for earth g = 9.8m/s = 32 ft/s (approx values)
1.2.3.2.3. Thus F1->2 = G m1 m2 / |r1-r2|2 = m (GM/R2) = mg (M is the mass and R is the radius of the earth)
1.2.3.2.4. Thus g = GM/R2 is the acceleration due to gravity.
1.2.3.2.5. The gravitational field is defined as the force on a unit mass: F/m = g = GM/R2
1.2.3.2.5.1. Show mapping of the gravitational field as the force on a unit mass
1.2.3.3. Advanced
1.2.3.3.1. Newton’s law of gravitation on m located at r: Fi->m = G mimi (ri-r) / |ri-r|3
1.2.3.3.2. Gravitational Field: : g(r) = G i mi (ri-r) / |ri-r|3 in units of acceleration m/s2
13
1.3. Solids, Fluids, & Waves
1.3.1.Elasticity <CJ chap 10.1, 10.7, 10.8 >
1.3.1.1. Discussion
1.3.1.1.1. When systems are distorted from equilibrium, the restoring force is proportional to the deformation
1.3.1.1.2. Generally: Stress is proportional to strain within the elastic limit:
1.3.1.1.2.1. Young’s Modulus: Stretch & Compression of solid: F/A = Stress & L/L0 is the strain
1.3.1.1.2.2. Shear modulus: Forces which create a shear of solid: F/A = Stress & X/L0 is the strain
1.3.1.1.2.3. Bulk modulus: Pressure on solids, liquids or gasses: P=F/A = Stress & V/V0 is the strain
1.3.1.2. Mathematical
1.3.1.2.1. Hookes Law: F = -k x where a force F causes a proportional deformation x from equilibrium
1.3.1.2.1.1. The constant k is called the ‘spring constant’
1.3.1.2.1.2. The potential energy stored in a deformed system is PEdeformaiton = ½ k x2 (=work to deform)
1.3.1.2.2. Young’s Modulus: F = Y A (L/L0) where Y is the Young’s modulus for that substance
1.3.1.2.2.1. and where A is the area where the force F is applied, and L0 is the original length
1.3.1.2.2.2. Examples of values are Brass: 9.0E10, Brick 1.4E10, Steel 2.0E11, Aluminum 6.9E10
1.3.1.2.2.3. Note that in some substances, Y for tension (pulling) is different from Y for compression
1.3.1.2.3. Shear modulus: F = S A (X/L0) where S is the shear modulus for that substance, F is applied force
1.3.1.2.3.1. A is the surface area, X the length of the shear, and L0 is the length of the applied shear
1.3.1.2.3.2. Examples of values are: Brass 3.5E10, Steel 8.1E10, Aluminum 2.4E10
1.3.1.2.4. Bulk modulus: P = -B (V/V0) where Pressure P = F / A in units of N/m 2 and B is the Bulk modulus
1.3.1.2.4.1. and V is the change in volume while V0 is the original volume
1.3.1.2.4.2. Examples of values are: Brass 6.7E10, Steel 1.4E11, Water 2.2E9, Ethanol 8.9E8
1.3.1.3. Advanced
1.3.1.3.1. The Taylor series expansion of the potential is V(x) = V(0) + dV/dx|x=0 x +1/2 d2V/dx2|x=0 x2 …
1.3.1.3.1.1. A solid material near equilibrium (x=0) has no force (thus dV/dx|x= =0) and we can set V(0)=0
1.3.1.3.1.2. Thus V(x) = ½ k x2 in lowest order approximation thus giving F = -kx
14
1.3.2.Simple Harmonic Motion <CJ chap 10 >
1.3.2.1. Discussion
1.3.2.1.1. Systems distorted from equilibrium and released (without friction), then oscillate about that equilibrium
1.3.2.1.2. This oscillation has a mathematical form of a sin or cos function and is called simple harmonic motion
1.3.2.2. Mathematical
1.3.2.2.1. Let a mass m, feel a spring force F= -kx where x is the distance from equilibrium. Then:
1.3.2.2.1.1. ma(t) = -kx(t) which has the solution x(t) = A cos(t + ) where = angular velocity
1.3.2.2.1.2. For this x(t) to be the solution, = k / m must hold
1.3.2.2.1.3. A is the amplitude of the oscillation since cos has a range from -1 to +1. It can assume any value
1.3.2.2.1.4. The phase of the oscillation = which can assume any value and is determined by x(t=0)
1.3.2.2.1.5. A complete cycle occurs by definition in time T and since cos has a cycle of 2, then =2
1.3.2.2.1.6. Consequently the period T =2This equation is important since it relates T (intuitive) and
1.3.2.2.1.7. Since the frequency f is the reciprocal of the period, f = 1/T , then f = 2
1.3.2.2.2. The importance of these results are that they describe ANY system near equilibrium (with no friction)
1.3.2.2.3. The derivation of these results requires calculus and the solution of the differential equations.
1.3.2.3. Advanced
1.3.2.3.1. Simple harmonic motion (motion of a mass m near equilibrium) is given by: ma(t) = -kx(t) –bv +Fext
1.3.2.3.1.1. Put as a differential equation we get: m d2x/dt2 + b dx/dt + kx = Fext where x = x(t)
1.3.2.3.1.2. This is one of the most important equations in physics and also is the RCLV circuit equation
1.3.2.3.1.3. It is a second order (second derivative is highest), linear, inhomogeneous (Fext ) differential eq
1.3.2.3.1.4. The general solution to the inhomogeneous equation (x gi(t)) is the general homogeneous (xgh(t))
plus any inhomogeneous solution xai(t) Thus: xgi(t) = xgh(t) + xai(t). We now find each of these.
1.3.2.3.2. Solution to the general homogeneous equation m d2x/dt2 + b dx/dt + kx = 0 for xgh(t)
1.3.2.3.2.1. The solution is of the form: xgh(t) = A et which we substitute into the equation to get:
1.3.2.3.2.2. m 2A et + b A et + k A et = 0 thus it follows that m2 + b + k =0 which is a quadratic eq.
1.3.2.3.2.3. Thus =
(b b 2 4mk ) /(2m) or with b / 2m and 0 k / m then we get
1.3.2.3.2.4.
2 02
1.3.2.3.2.5.
There are three types of solutions depending upon and 0 :
as the condition for xgh(t) = A et to be the general homogeneous solution.
1.3.2.3.2.5.2.
Overdamped: > 0 then x Ae( 0 )t Be(
t
t
Critically damped: = 0 then x Ae Bte
1.3.2.3.2.5.3.
Underdamped: < 0 then defining 02 2 we get
2
1.3.2.3.2.5.1.
2
2 02 )t
x Ae t it Be t it Ae t cos(t ) where A and replace A & B as the constants
1.3.2.3.2.5.4. Description of each solution & degenerate case
1.3.2.3.2.6. Inhomogeneous force that is constant: F = F0 is solved by adding F0/k to solution xgh(t)
1.3.2.3.2.7. Inhomogeneous oscillatory force F = F0 ei1t can be solved with xai(t) = X ei1t & solve for X:
1.3.2.3.2.7.1. Upon substitution we get [m(i1)2 +b(i1) +k]Xei1t =F0 ei1t
1.3.2.3.2.7.2. Solving for X we get X = (F0/m) / ([ (i1)2 +(b/m)(i1) +k/m] thus using & 0 we get:
1.3.2.3.2.7.3. X = (F0/m) / ((02 -12)+i21) where we must put the complex number in normal form:
1.3.2.3.2.7.4. (u+iv)-1 = (u-iv)/ (u2+v2)-1/2 which we put into the form Rei with R = (u2+v2)-1/2 and
1.3.2.3.2.7.5.
thus R = ((02 -12)2+ (21)2)-1/2
tan-1 (-v/u) = tan-1 (21/(12 -02)) where the ‘-‘ sign was put on the lower term.
This gives the final result that
xai(t) = R ei1t + i
Resonance can be easily seen as maximizing the amplitude R when 0 =1
This occurs when the applied force is at the same frequency as the natural frequency 0
Likewise one can see the phase shift between the response xai(t) and the applied force.
The general solution is then the sum of these two solutions xgi(t) = xgh(t) + xai(t).
The homogeneous solution xgh(t) is called the transient as the term e-t decays with time.
The inhomogeneous solution is called the steady-state solution as it persists in time.
Generality of the application of these results:
As shown here, we have derived the response of a mechanical system near equilibrium
But the same solution also applies exactly to planetary motion
It also provides the general solution to an RLC circuit with a sinusoidal applied voltage.
Thus these methods are of the greatest importance in physics.
15
1.3.3.Fluids <CJ chap 11 >
1.3.3.1. Discussion
1.3.3.1.1. Fluid Flow:
1.3.3.1.1.1. Steady Flow : the velocity is constant at each point in the fluid
1.3.3.1.1.2. Unsteady Flow: the velocity changes at a given point with time
1.3.3.1.1.3. Turbulent Flow: the velocity changes randomly and erratically in both magnitude & direction
1.3.3.1.1.4. Compressible: density of the fluid changes as pressure changes
1.3.3.1.1.5. Incompressible: the density of the fluid (essentially all liquids) is constant when pressure changes
1.3.3.1.1.6. Viscous Flow: Flow is impeded by loss of energy resisting the flow
1.3.3.1.1.7. Nonviscous Flow: Flow is smooth and non-resistive with no (or little) energy loss
1.3.3.1.1.8. Ideal Fluid = a Nonviscous incompressible fluid (water is a fair example)
1.3.3.1.1.9. Streamline Flow = The streamlines (trajectories of flow) are steady, constant velocity at one point
1.3.3.2. Mathematical
1.3.3.2.1. Mass Density per unit volume of a substance is defined by = m/V with units of kg/m 3
1.3.3.2.1.1. Examples of mass density: Brass 8470; Gold 19,300; Lead 11,300; Mercury 13600; Water 1,000
1.3.3.2.1.2. Also Wood 550; Ice 917; Aluminum 2,700; Air 1.29; Helium 0.18; Hydrogen 0.09; Oxygen 1.43
1.3.3.2.2. Specific Gravity = Density of substance / Density of water at 4 degC (ie 1,000 kg/m 3)
1.3.3.2.3. Pressure is defined by P = F/A with units of Pascal = Pa= N/m 2
1.3.3.2.3.1. Atmospheric pressure at sea level is 1.013E5 Pa
1.3.3.2.3.2. Pressure in a fluid P = Psurface + gh (derive by Psur A + hA) g = P A then divide by A)
1.3.3.2.3.3. Pressure gauges (water & Hg columns supported)
1.3.3.2.3.4. Gauge pressure in a manometer: height is proportional to the difference of pressures
1.3.3.2.3.5. Pascal’s principle: the change in pressure applied to an enclosed fluid is transmitted to all parts
1.3.3.2.3.5.1. F1 / A1 = F2 / A2 can be used to lift a heavy object (car) as a hydraulic lift
1.3.3.2.4. Archimedes’ (287-212 BCE) Principle: Fbuoyant = W fluid displaced
1.3.3.2.5. Equation of Continuity relates the mass flow rate at two points in the fluid
1.3.3.2.5.1. Is equivalent to the conservation of mass
1.3.3.2.5.2. 1A1v1 = 2A2v2 (ie is conserved from one point to another)
1.3.3.2.6. Bernoulli’s (1700-1782) Equation governs the steady nonviscous incompressible fluid flow
1.3.3.2.6.1. Is equivalent to conservation of energy
1.3.3.2.6.2. P1 +1/2v12 + gy1 = P2 +1/2v22 + gy2 (ie is conserved from one point to another)
1.3.3.2.7. Viscous Flow describes the Force needed to move a layer of viscous fluid at constant velocity
1.3.3.2.7.1. F=Av / y where = the coefficient of viscosity with units of Pa s (also 1 poise = 0.1 Pa s)
1.3.3.2.7.1.1. where A is the area of the fluid, v is its velocity, and y is distance from immovable plane
1.3.3.2.7.2. Poiseuille’s law gives the volume flow rate Q in a pipe of radius R, length L, and pressures P 1, P2
1.3.3.2.7.2.1. Q = dV/dt = R4 (P2 –P1) /(8L)
1.3.3.3. Advanced
16
1.3.4.Mechanical Waves & Sound <CJ chap 16 >
1.3.4.1. Discussion
1.3.4.1.1. A wave is a traveling disturbance in a medium that carries energy but not mass
1.3.4.1.2. Fourier’s theorem All wave disturbances are (linear) combinations of sin & cos waves of different freq
1.3.4.1.3. Core concepts concerning waves:
1.3.4.1.3.1. The period, T, is the time required for one full cycle of the wave
1.3.4.1.3.2. The frequency, f, is the number of compete cycles per unit time (second): Hertz = Hz =Cycles/s
1.3.4.1.3.3. The amplitude, A, of the wave is the maximum displacement from equilibrium
1.3.4.1.3.4. The wavelength, is the (shortest) length in meters between two identical parts of the wave
1.3.4.1.3.5. The phase, , of the wave is the angle in radians that the wave is displaced in the sin or cos
1.3.4.1.3.6. The angular velocity = 2 f
1.3.4.1.3.7. The wave number k = 2
1.3.4.1.4. Objective (physically measurable) aspects of sound verses Subjective (perceived by human senses)
1.3.4.1.4.1. Intensity of the wave (in Watts / m 2) verses Loudness (measured in decibels)
1.3.4.1.4.2. Frequency (Hz) verses the perceived frequency or Pitch
1.3.4.1.4.3. Harmonic Structure (composition of overtones or harmonics) verses the Quality
1.3.4.1.5. Standards:
1.3.4.1.5.1. Musical frequency: A above middle C is 440 Hz and is the standard of western music
1.3.4.1.5.2. The standard for acoustics and sound for human hearing is 1,000 Hz = 1KHz
1.3.4.1.5.3. The normal maximum range of human hearing is 20Hz to 20KHz
1.3.4.1.5.4. Velocity of sound is 331 m/s at 0 C and increases by 0.6 m/s for each degree C
1.3.4.1.5.5. V of sound in substances m/s: Steel 5,960; Glass 5,640; Water 1,482; Helium 965
1.3.4.1.6. Very Important: The human body responds to sound intensity, frequency, light intensity, heat,
pressure and other stimulations as the log of the stimulus. This allows a person to have a vast range of
sensing without overloading the senses at high values and still be extremely sensitive to low values.
1.3.4.1.6.1. For example sound intensity is measured in log I/I0 and the piano scale is the log of the frequency
1.3.4.1.6.2. It is perhaps a deep concept that information is measured as the logarithm of a probability
1.3.4.1.6.3. Perhaps life forms take the sensory log to automatically measure the maximum information
1.3.4.2. Mathematical
1.3.4.2.1. Important equation: f = v for any wave where f= frequency, wave length, v = wave velocity
1.3.4.2.2. Also fundamental is the relationship: f = 1/T
1.3.4.2.2.1. Example of a radio wave: f = 102 MHz, c = 3E8 m/s thus = 1.02E8/3E8 = 0.34 m
1.3.4.2.2.2. Example of a sound wave: f = A 440 Hz, vsound = 1100 ft/s thus = 2.5 ft
1.3.4.2.3. Velocity of a wave on a string = vstring = (F / (m/l))1/2 where (m/l) = the mass per unit length
1.3.4.2.4. Equation for wave motion: y(x,t) = A cos(t – kx + )
1.3.4.2.5. Loudness is measured in decibels (dB) = 10 log(I/I0) where I = intensity in w/m 2,
1.3.4.2.5.1. I0 = 10-12 w/m2 is the threshold of human hearing
1.3.4.2.5.2. An increase of 10 dB is perceived as twice the loudness
1.3.4.2.6. Doppler shift in frequency results when a source is moving vs or the observer is moving at vo
1.3.4.2.6.1. Observer moves toward source: f0 = fs (1-v0/v)/(1+vs/v) & away from source f0 = fs (1+v0/v)/(1-vs/v)
1.3.4.3. Advanced
17
1.3.5. Linear Superposition of Waves, Interference, & Music <CJ chap 17 >
1.3.5.1. Discussion
1.3.5.1.1. Linear Superposition: The total wave amplitude at a point is the sum of the separate arriving waves
1.3.5.1.1.1. Constructive Interference: When both waves are additive & become greater than separately
1.3.5.1.1.2. Destructive Interference: When the two waves are of opposite signs and thus partly cancel
1.3.5.1.1.3. If a wave proceeds by two paths, the phase difference due to path length can lead to interference
1.3.5.1.2. Direction of wave vibration relative to motion distinguishes two types of waves:
1.3.5.1.2.1. Transverse waves: where the medium vibrates perpendicular to the velocity
1.3.5.1.2.1.1. e.g. EM waves including light as E & M are orthogonal to v & surface water waves
1.3.5.1.2.2. Longitudinal waves: where the media vibrates parallel to the velocity
1.3.5.1.2.2.1. e.g. sound (compression) waves
1.3.5.1.2.3. Torsion waves, a third type, are very rare and consists of a twisting wave about v
1.3.5.2. Mathematical
1.3.5.2.1. Interference occurs between a wave and itself dependent upon the paths taken x :
1.3.5.2.1.1. Constructive interference: x = n where n =
1.3.5.2.1.2. Destructive interference: x = (n+1/2) where n =
1.3.5.2.2. Interference of a single slit of width D: Angle to respective maxima is sin/D (=1.22/D circular)
1.3.5.2.3. Interference of two nearby frequencies f1 & f2 results in the average frequency modulated by beats:
1.3.5.2.3.1. One hears ½(f1 + f2) * ½ (f1 - f2) = average frequency * beats with frequency ½ (f1 - f2)
1.3.5.2.3.1.1. These ‘beats’ are really modulations (oscillations) in the amplitude of the average freq.
1.3.5.2.3.2. Since the ‘frequency’ ½ (f1 - f2) has two maxima per cycle, one gets a beat period of T=1/(f1 - f2)
1.3.5.2.4. String (and air column) vibrations
1.3.5.2.4.1. Stretched strings of length L can sustain vibrations that have an integer number of half waves in L
1.3.5.2.4.2. Thus with a node at each end (the attached point cannot move) we get n(L
1.3.5.2.4.3. Thus the frequencies for each integer n are given by: fn = v/ = n v/(2L) = n f1 thus multiples of f1
1.3.5.2.4.4. Air columns that are closed at both ends have nodes there and thus obey the same equation.
1.3.5.2.4.5. If an air column is open at one end, one has an antinode thus one must have (n odd/4) = L
1.3.5.2.4.6. Thus : fn = v/ = nodd v/(4L) = nodd f1 where nodd = 1, 3, 5, 7, ….
1.3.5.2.4.7. These values of n refer to the ‘nth’ harmonic or to the (n-1)th overtone where n=1 is fundamental
1.3.5.2.4.8. Thus the 5th harmonic is 4th overtone; and the 1st harmonic is the fundamental.
1.3.5.2.5. Musical Frequencies:
1.3.5.2.5.1. Two notes sound ‘consonant’ when their frequencies are simple integer multiples (Pythagoras)
1.3.5.2.5.2. Unison (same note) is 1/1, an octave is 2/1, a fifth is 3/2; and a fourth is ¾ in order of consonance
1.3.5.2.5.3. When a string is plucked or air column sounded the frequencies = integers times the fundamental
1.3.5.2.5.4. Pythagoras tuned early instruments by going up a fifth, down a fourth, up a fifth, etc
1.3.5.2.5.5. An improved method was invented by JS Bach called equitempered tuning (all half steps equal)
1.3.5.2.5.6. Since there are 12 half steps in an octave in western music, each half step goes up by a factor
1.3.5.2.5.7. Thus the notes are f1, f1, 2 f1, ….12 f1 which must = 2 f1 (an octave) thus
1.3.5.2.5.8. This is the ratio of two adjacent notes a half step apart in music.
1.3.5.2.5.9. The standard that fixes all the notes is A440 = 440 Hz which is the A above middle C
1.3.5.3. Advanced
1.3.5.3.1. Just discernable differences in frequency. At 1,000 Hz & higher one can discern a 0.5% freq change
1.3.5.3.1.1. A ‘cent’ = 1/100 of a half step. One can discern a frequency difference of about 5 cents.
1.3.5.3.1.2. Just as an half note ratio is 21/12 , the cent is the ratio 21/1200 = 1.00057779
1.3.5.3.2. Just discernable differences in loudness, although varying with freq etc, is about 1.0 dB
1.3.5.3.3. Differences between the equitempered frequencies and ‘just’ or ‘perfect ratios of intervals
1.3.5.3.4. Reverberation Time = Time for the sound intensity level to reduce to 1E-6 (60dB) of original value
1.3.5.3.4.1. T(s) = 0.049 V/A where V (ft3) = volume of the room and A = area of an absorbing ‘hole’ (ft2)
1.3.5.3.4.2. The perfectly absorbing hole area, A = ai Si where ai is the absorption coef. of an area of Si ft2
1.3.5.3.4.3. Approximate T values in sec are: Speech 0.4 to 0.8; music 1 to 1.6, etc
1.3.5.3.4.4. Absorption values at 1kHz are ai = Marble 0.01; Plate glass 0.04; Plywood on studs 0.10;
1.3.5.3.4.4.1. Carpet 0.37; Plaster 0.10; Acoustical plaster 0.78; Each person 7.0; Empty cloth seat 5.0
1.3.5.3.5. Perfect frequency ratios & the Equitempered value: Fifth (3/2 , 1.49831), Fourth (4/3, 1.33484),
1.3.5.3.5.1. Maj Third (5/4, 1.25992), Min Third (6/5, 1.18921), Maj Six (5/3, 1.68179), Min Six (8/5, 1.58740)
18
2. Thermodynamics
2.1.1.Temperature & Heat <CJ chap 12 >
2.1.1.1. Discussion
2.1.1.1.1. Temperature: a measure of the average random energy in a substance. Units: temperature scales
2.1.1.1.1.1. Fahrenheit scale: 0 F: freezing sea water, 100 F: for human body, then 32 F: freezing water
2.1.1.1.1.2. Celsius scale: 0 C: freezing water, 100 C: for boiling water then -273.15 = absolute zero
2.1.1.1.1.3. Kelvin scale: by definition
K = 273.15+ C.
2.1.1.1.1.4. All scales are defined in terms of K where 0 K is absolute zero & 273.16 K = water triple point
2.1.1.1.2. Thermometers
2.1.1.1.2.1. Use the ‘linear’ expansion of a substance such as mercury with temperature
2.1.1.1.2.2. Optimal thermometer is the constant volume gas thermometer of an ‘ideal gas’
2.1.1.1.3. Heat is random (mostly kinetic) energy in a substance – the energy that flows due to temperature diff.
2.1.1.1.3.1. Units of heat are in Joules (J)
2.1.1.1.3.2. 1 Calorie = amt of heat needed to raise the temperature of 1 kg of water 1 C
2.1.1.1.3.2.1. The Calorie (upper case) = 1000 calories which pertain to a gram of water not kg
2.1.1.1.3.2.2. It is the Calorie or Kilocalorie that we eat when we eat food (energy)
2.1.1.1.3.3. 1 BTU = amt of heat needed to raise the temperature of 1 pound of water 1 F
2.1.1.1.3.4.
2.1.1.2. Mathematical
2.1.1.2.1. Temperature conversion: F = 32 +C*9/5, C = (F-32)*5/9, K = C + 273.15
2.1.1.2.2. Linear thermal expansion of a solid: Change L in length L0 due to a change in temperature is
2.1.1.2.2.1. L = L0 T where is the coefficient of linear expansion in 1/C
2.1.1.2.2.2. Examples: Brass 19E-6; Gold 14E-6; Glass 8.5E-6; Aluminum 23E-6
2.1.1.2.3. Volumetric Expansion of a solid or liquid: Change V in length V0 due to a change in temperature
2.1.1.2.3.1. V = V0 T where is the coefficient of volume expansion in 1/C
2.1.1.2.4. Heat raises the temperature of a substance (except during a phase change) by :
2.1.1.2.4.1. Q = c m T where c is the specific heat of the substance
2.1.1.2.4.2. Examples of c (J/(kg C): Water 4186; Mercury 139; Aluminum 900; Glass 840; Lead 128;
2.1.1.2.5. The heat Q required for a phase change is Q = m L where m = mass and L is the latent heat
2.1.1.2.5.1. Latent heat of fusion, Lf, refers to melting or freezing (J/kg)
2.1.1.2.5.2. Latent heat of vaporization, Lv, refers to boiling or condensation (J/kg)
2.1.1.2.5.3. Lf & Lv in (J/kg): Water 33.5E4, 22.6E5; Gold 6.28E4, 17.2E5; Nitrogen 2.60E4, 2.00E5
2.1.1.2.5.4. Tmelt & Tboil in Celcius: Water 0, 100; Gold 1063, 2808; Nitrogen -210.0, -195.8
2.1.1.3. Advanced
19
2.1.2.Transfer of Heat <CJ chap 13 >
2.1.2.1. Discussion
2.1.2.1.1. Convection: the process of conveying heat from one point to another by the movement of fluid
2.1.2.1.1.1. Distinguish natural convection or forced convection
2.1.2.1.1.2. The formulas for convection are extremely complex and nonlinear as they are fluid flows
2.1.2.1.1.3. So at this level we do not attempt to discuss the mathematical aspects of convection
2.1.2.1.2. Conduction: heat is transferred through a material without motion of the material itself
2.1.2.1.2.1. Distinguish thermal conductors from thermal insulators
2.1.2.1.2.2. The formulas for conduction in solids is simple and of great importance
2.1.2.1.3. Radiation: the process by which electromagnetic radiation (cavity radiation) is emitted
2.1.2.1.3.1. The profile of emitted radiation is dependent upon the temperature of the object
2.1.2.1.3.2. We are familiar with substances that emit infrared (heat) because they are hot
2.1.2.1.3.3. We are also familiar with much hotter objects that glow red hot, or white or even blue.
2.1.2.1.3.4. The formula for radiation is also relatively simple but unusual as we will see below.
2.1.2.2. Mathematical
2.1.2.2.1. Conduction heat/time Q/t = k A T / L where k= thermal conductivity, A=area, L=thickness T=temp
2.1.2.2.1.1. Thus: Q/t = A T / (L/k) = A T / R where R = L/k is called the R factor (combines k & L)
2.1.2.2.1.2. R factors are additive for building materials and with units of BTU/hr for Q/t, and A in ft2, T
2.1.2.2.1.3. Values are: R = 1 glass, 2 double pane; R=11 for 3.5” wall insul, R=19 for 6” floor/attic insul
2.1.2.2.1.4. and R= about 3.4 for uninsulated walls, floors, and ceilings .
2.1.2.2.1.5. Problems involving building materials allow the R factors to simply add to obtain the total.
2.1.2.2.2. Radiation (Stefan Boltzman law): Q/t = A T4 where is emissivity (1 black, 0 shiny metal)
2.1.2.2.2.1. = Stefan Boltzman constant = 5.67E-8 (J/(s m2 K4)), and A is the area in m 2
2.1.2.3. Advanced
20
F
2.1.3.Ideal Gas Law & Kinetic Theory <CJ chap 14 >
2.1.3.1. Discussion
2.1.3.1.1. Atomic Mass Unit = 1.6605E-27 kg = 1/12 of the mass of 12C (as this is the best reference)
2.1.3.1.2. Mole = the number of entities equal to the number of atoms in 12 grams of 12C
2.1.3.1.2.1. Mole = Avogadro’s number = NA = 6.022E23
2.1.3.1.2.2. Thus Avogadro’s number of entities (ie one mole) of a chemical is its molecular mass in grams
2.1.3.1.2.3. Thus 18 grams of H20 is one mole and contains NA molecules
2.1.3.1.3. Ideal gas is a gas of low density, point particles with no internal freedoms, and elastic collisions
2.1.3.2. Mathematical
2.1.3.2.1. Ideal gas law P V = n R T (P=Pressure, V=Volume, n= number of moles, T = temp. in K )
2.1.3.2.1.1. and R is the Universal Gas Constant 8.31 J/(mole*K)
2.1.3.2.1.2. Equivalently one can write PV = (n* NA ) (R/ NA) T = N k T where N = Number of molecules and
2.1.3.2.1.3.
k = R/ NA the Boltzman constant = 1.38E-23 J/K
2.1.3.2.1.4. Boyles law (constant T) gives P1V1 = P2V2 used to compare a gas ‘before and after’ follows
2.1.3.2.1.5. Charles law (constant P) gives V1 /T1 = V2/T2
2.1.3.2.2. Using kinetic theory one can show that PV = (2/3) N <KE> thus when combined with the ideal gas law
2.1.3.2.2.1. we get that the average kinetic energy is <KE> = (3/2) k T thereby interpreting temperature
2.1.3.2.2.2. Also the internal energy U = N <KE> thus U = (3/2) N k T = (3/2) n R T for a monoatomic gas
2.1.3.2.3. Diffusion – Fick’s Law of Diffusion m/t = (D A C) / L = mass per time diffusing in a solvent
2.1.3.2.3.1. where C is the concentration difference, in a channel of length A and cross section area A
2.1.3.2.3.2. The diffusion constant D for water vapor in air is 2.4E-5 m2/s
2.1.3.3. Advanced
2.1.3.3.1. Derive <KE> = (3/2) k T from basic kinetic theory
21
2.1.4.Thermodynamics <CJ chap 15 >
2.1.4.1. Discussion
2.1.4.1.1. Laws of thermodynamics:
2.1.4.1.1.1. 0th law: Two systems in equilibrium with a third system will be in equilibrium with each other
2.1.4.1.1.2. 1st law: The change in internal energy is equal to the heat gained minus the work done
2.1.4.1.1.2.1. This is the law of conservation of energy including heat in the equation
2.1.4.1.1.3. 2nd law: Heat flows spontaneously from a higher T to one of lower T, never conversely
2.1.4.1.1.3.1. or: The total entropy (disorder) always increases for an irreversible process and
2.1.4.1.1.3.1.1. entropy is constant for a reversible process.
2.1.4.1.1.4. 3rd law: It is not possible to lower system temperature to absolute zero in a finite number of steps
2.1.4.1.2. Types of processes named
2.1.4.1.2.1. Isobaric means that pressure is kept constant (P = 0)
2.1.4.1.2.2. Isothermal means that temperature is kept constant (
2.1.4.1.2.3. Isochoric (or isovolumetric) means that the volume is kept constant (V = 0)
2.1.4.1.2.4. Adiabatic process is one in which there is no change (flow) of heat (Q = 0)
2.1.4.2. Mathematical
2.1.4.2.1. 1st Law of thermodynamics: The change in internal energy = U = Q - W where
2.1.4.2.1.1. Q is the heat input into the system and W is the work done by the system
2.1.4.2.2. For an isobaric process (P = 0), the work done is W = P V
2.1.4.2.3. For an isothermal quasi-static ideal gas process W = n R T ln(Vf/Vi)
2.1.4.2.4. For an adiabatic (Q = 0) quasi-static process W = (3/2) n R (Ti - Tf) for n moles of a monoatomic gas
2.1.4.2.5. Also for an adiabatic ideal gas: P Vi = P Vfwhere = cp / cv
2.1.4.2.6. Specific heat capacities: Recall Q = C T where C is the specific heat:
2.1.4.2.6.1. CP = (5/2) R for a monatomic ideal gas at constant pressure and Cv = (3/2) R at constant volume
2.1.4.2.6.2. CP = (7/2) R for a diatomic ideal gas at constant pressure and Cv = (5/2) R at constant volume
2.1.4.2.6.3. For any type of ideal gas CP - CP = R
2.1.4.2.7. Heat Engines take in heat Q and output useful work W with an efficiency = W/Q
2.1.4.2.7.1. but since Qh = W + Qc then = W/Qh = 1 - Qc/Qh (all terms are positive magnitudes)
2.1.4.2.7.2. For a Carnot engine: Qc/Qh = Tc/Th thus carnot = 1 - Tc/Th
2.1.4.2.8. Coefficient of Performance (COP) for refrigerators and heat pumps:
2.1.4.2.8.1. COPref = Qc / W
and COPhp = Qh / W
2.1.4.2.9. Entropy changes S in which heat enters or leaves a system reversible at constant T is given by:
2.1.4.2.9.1. S = Q/T
2.1.4.2.9.2. Entropy is a measure of the system disorder
2.1.4.3. Advanced
22
3. Electromagnetic Theory
3.1. Electricity
3.1.1.Electric Forces <CJ chap 18.1-18.5 >
3.1.1.1. Discussion
3.1.1.1.1. We are all familiar with static electricity, lightning, and electrical currents from an early age.
3.1.1.1.2. Today we are familiar with the sources of charge: electrons, protons, ions, and atomic structure.
3.1.1.1.2.1. What is electrical charge? We do not really know – it is an intrinsic property like mass.
3.1.1.1.3. Electric charges are + & - Like charges (++ and - -) repel while opposites (+ -) attract.
3.1.1.1.3.1. Benjamin Franklin (1706-1790) defined charge & related it to lightning
3.1.1.1.4. Charges are quantized in integer multiples of the basic charge e = 1.6E-19 C
3.1.1.1.4.1. Robert Milliken proved this in 1909 and measured the charge on the electron e3.1.1.1.5. Electric charge is measured in units of Coulombs
3.1.1.1.6. The total electric charge in a closed domain is conserved
3.1.1.1.7. Conductors allow charges to move freely. Other materials are called insulators.
3.1.1.1.8. Coulomb’s law discovered 1785 By Charles Coulomb using a torsion balance to determine Fc
3.1.1.1.9. Electric Induction Charging – a conductor attached to the ground is ‘grounded’ / Contact charging
3.1.1.1.10. Linear Superposition: electrical (and magnetic) forces are (vectorially) additive from individual forces
3.1.1.2. Mathematical
3.1.1.2.1. Coulomb’s Law for forces between charges:
3.1.1.2.1.1. F1-2 = keq1 q2 / r2 where ke = 9E9 = 1/(40) exactly = 8.9875 E9
3.1.1.2.1.2. The constant 0 is the permittivity of the vacuum
3.1.1.2.1.3. Force F is measured in Newtons
3.1.1.2.1.4. Charge per unit volume = Q/V, per unit area = Q/A, & per length= Q/l
3.1.1.2.2. Problems with two charges
3.1.1.2.3. Vector problems with multiple charges
3.1.1.3. Advanced
3.1.1.3.1. Vector Statement of Coulomb’s Law
3.1.1.3.1.1. F1->2 = keq1 q2 (r2-r1) / |r2-r1|3 where F and r are vectors
3.1.1.3.2. Generally the force on a charge q from other charges is Fq(r) = q I qi (r-ri) / |r-ri|3 thus:
3.1.1.3.3. E(r) = i qi (r-ri) / |r-ri|3 = Fq(r) /q
3.1.1.3.4. E has units of Newtons / Coulomb (there is no special name for this unit)
3.1.1.3.5. Vector problems
23
3.1.2.Electric Field <CJ chap 18.6-18.8 >
3.1.2.1. Discussion
3.1.2.1.1. Force at a distance was difficult for people to accept – thus the electric field, E, was ‘invented’
3.1.2.1.2. The electric field at a point is the force a unit charge would experience.
3.1.2.1.3. E(x,y,z,t) is a vector field.
3.1.2.1.4. Electric field lines display E . (E was at first an imaginary concept.)
3.1.2.1.5. They can never cross. They begin at + and end at – charges.
3.1.2.1.6. E is zero inside a conducting material and excess resides on the surface.
3.1.2.1.7. E just outside a conductor is always perpendicular to the conductor’s surface.
3.1.2.1.8. Charge accumulates where the surface has the smallest radius of curvature.
3.1.2.1.9. The electric field outside of a charged sphere shell is as though all charge is at its center
3.1.2.1.10. The electric field of a charged spherical shell is zero (inside the sphere) - shielding
3.1.2.1.11. Electric dipole is a pair of equal but opposite charges separated by a short distance
3.1.2.1.11.1.
Some molecules are dipolar such as water
3.1.2.1.11.2.
The electric field of a dipole is similar to that of a magnetic dipole (magnet)
3.1.2.1.12. The electric field inside a parallel plate capacitor is uniform & often used as a source of an E field.
3.1.2.2. Mathematical
3.1.2.2.1. Electric field equations
3.1.2.2.2.
E = F/q =kq0/r2 thus F = q E
3.1.2.2.3. The electric field of a dipole (+ -), and the electric fields of the pairs ( + +) or (- -)
3.1.2.2.4. Motion of a charged particle in a constant E field. ma = qE, use “constant a” formulas
3.1.2.2.5. Electric dipole moment p is defined as p = Qd where +Q and –Q are a distance d apart
3.1.2.2.5.1. The electric dipole p is a vector pointing along d from the negative to the positive charge
3.1.2.2.5.2. An electric dipole feels a torque in an electric field of = p x E where is a vector
3.1.2.2.5.3. An electric dipole in a field E has an energy of U = - p E where U is a scalar
3.1.2.3. Advanced
3.1.2.3.1. Vector expression of the electric field
3.1.2.3.2. E(r) = k q1 (r-r1) / |r-r1|3 where E and r are vectors
3.1.2.3.3. Generally the Electric field from charges qi is Eq (r) = i qi (r-ri) / |r-ri|3
3.1.2.3.4. Vector problems
24
3.1.3. Gauss’ Law <CJ chap 18.9 >
3.1.3.1. Discussion
3.1.3.1.1. Gauss’ law can be used to compute the electric field in symmetric cases.
3.1.3.1.2. For a conductor:
3.1.3.1.3. The electric field is zero everywhere inside a conductor thus conductors can be used to shield
3.1.3.1.4. Any excess charge resides on the surface of the conductor
3.1.3.1.5. On an irregular shaped conductor, charge accumulates where the radius of curvature is the smallest.
3.1.3.2. Mathematical
3.1.3.2.1. Derivations from Gauss’ law
3.1.3.2.1.1. Plane: E = /(20)
3.1.3.2.1.2. Line charge: E= 0r)
3.1.3.2.1.3. Inside a parallel plate capacitor: E = /(0) and is uniform
3.1.3.2.1.4. E = 0 = Also just outside a conductor
3.1.3.3. Advanced
3.1.3.3.1. Gauss’ Law:
3.1.3.3.1.1. The electric flux
3.1.3.3.1.2. Thus
3.1.3.3.2.
d through a closed surface
dqinside /0
Derive Gauss’ law from Coulomb’s
25
= qinside /0
3.1.4.Electric Potential & Potential Energy <CJ chap 19.1-19.4 >
3.1.4.1. Discussion
3.1.4.1.1. The potential energy of a system of charges is the work necessary to assemble them from infinity
3.1.4.1.1.1. The potential energy, U, is a scalar and is measured in units of Joules
3.1.4.1.2. The electric potential V(r), is the work needed to bring a unit charge to a point from infinity
3.1.4.1.2.1. V(r) is also a scalar and is measured in units of Volts = Joule / Coulomb
3.1.4.1.2.2. The plotting of the equal potential lines V(r) = constant for a system displays contours of V
3.1.4.1.2.3. These contours are always exactly perpendicular to the electric field E lines everywhere
3.1.4.1.2.4. In fact E is equal to (the negative of ) the gradient (rate and direction of maximum change) of V
3.1.4.1.2.5. Constant V(r) curves are good visual representations of the electrostatic environment, as is E
3.1.4.1.2.6. It is most common to consider changes in V (voltage differences) rather than absolute values
3.1.4.2. Mathematical:
3.1.4.2.1. Potential Energy = U = k q1 q2 / |r1 - r2| = Work needed to bring q1 & q2 from an infinite distance
3.1.4.2.1.1. The units of potential energy here are Joules. Note that U is a scalar not a vector.
3.1.4.2.1.2. The potential energy of several charges, qi is given by U = ½ k qi qj / |ri-rj|
3.1.4.2.1.2.1. Note the ½ arises from double counting in the summation over i and j
3.1.4.2.2. Electric Potential = V(r) = U/q0 = the work needed to bring a unit charge q0 from infinity to the point r
3.1.4.2.2.1. Thus V(r) = k q / r at r due to a charge q at the origin
3.1.4.2.2.2. The units of electric potential are given in Volts = Joules / Coulomb (or V=J/Q)
3.1.4.2.2.3. Usually, we look at voltage differences such as the potential difference between battery terminals.
3.1.4.2.3. Equipotential lines (curves that follow equal potential values) are perpendicular everywhere to E
3.1.4.2.3.1. These equipotential curves can be compared to isotherms (temperature) or isobars (pressure).
3.1.4.3. Advanced:
3.1.4.3.1.
Potential Energy = Work = dU = F dr = -q1
E dr = -k q1 q2
dr12 / r122
3.1.4.3.1.1. Thus U = k q1 q2 / r12 where r12 = |r1-r2| and when the integral goes from infinity up to r12
3.1.4.3.1.2. The units of potential energy U are in Joules and U is a scalar as it is a dot product
3.1.4.3.2. Electric Potential = V = U/q or for a single charge at the origin, V(r) = k q / r
3.1.4.3.2.1. The units of V are in Volts (V) where V=J/Q
3.1.4.3.2.2. Since V = -
E dr then it follows that Ex = - V / x and generally that E = - V
3.1.4.3.2.3. One recalls that ( / x, / y, / z )
26
3.1.5.Capacitance <CJ chap 19..5-19.7 >
3.1.5.1. Discussion
3.1.5.1.1. Given any two neutral conductors that are separated, say A and B, then carry a charge Q from A to B
3.1.5.1.1.1. A potential difference of V volts between A and B will result from this action.
3.1.5.1.1.2. If 2Q, 3Q etc is moved from A to B then 2V, 3V etc will be the resulting voltage difference.
3.1.5.1.1.3. This constant ratio of Q/V depends upon the geometry and is defined as the capacitance C =Q/V
3.1.5.1.2. Capacitors were the earliest methods of storing charge, voltage, and electrical energy.
3.1.5.1.3. The unit of capacitance is the Farad (F) = Coulomb/Volt (Q/V)
3.1.5.2. Mathematical
3.1.5.2.1. C = Q / V <Farad (F) = Coulomb / Volt >
3.1.5.2.2. Of a parallel plate capacitor C = q/V = A / (Ed) = A / ((0)d) or C = 0 A/d
3.1.5.2.3. Combinations of capacitors:
3.1.5.2.3.1. In parallel Ctotal = C1 + C2 + …. Cn
3.1.5.2.3.2. In series
1/Ctotal = 1/C1 + 1/C2 + …. 1/Cn
3.1.5.2.4. Energy stored in a capacitor W = ½ Q V = ½ C V2
3.1.5.2.5. Dielectric material
3.1.5.2.5.1. If a dielectric material is placed in a capacitor then V=V0 /
3.1.5.2.5.2. Where = / 0 dielectric constant = 1 for vacuum or air, 3.7 paper, 80 water…
3.1.5.2.5.3. It follows that C = C0
3.1.5.3. Advanced
3.1.5.3.1. Capacitance values for simple geometries:
3.1.5.3.1.1. A charged sphere of radius R: C = 4o R
3.1.5.3.1.2. Parallel plates of area A and separation d : C = o A /d
3.1.5.3.1.3. Cylindrical capacitor of length l and inner & outer radii a & b : C = l / [2 k ln (b/a)]
3.1.5.3.1.4. Spherical capacitor of inner and outer radii a & b: C = ab /[k (b-a)]
27
3.1.6. Electric Current & Resistance <CJ chap 20.1-20.7 >
3.1.6.1. Discussion
3.1.6.1.1. Electrical current is defined as the amount of charge in Coulombs that flows per second past a point
3.1.6.1.1.1. The unit of electrical current is the Ampere = Coulomb / Second or A =C/s
3.1.6.1.1.2. Electrical current flows because of a potential difference between two points in a material
3.1.6.1.2. There is resistance to all flow of electrical current except in superconductors.
3.1.6.1.2.1. One finds that the ratio of the voltage, to the current that flows, is a constant called the resistance
3.1.6.1.2.2. Electrical resistance is measured in Ohms = Volts / Ampere = V/A
3.1.6.2. Mathematical
3.1.6.2.1. Electric current = I = Q / t <Ampere (A) = Coulomb / second>
3.1.6.2.2. Ohm’s law:
R = V/I <Ohm = Volt / Ampere> is generally constant thus V = IR
3.1.6.2.3. Resistors in series & parallel:
3.1.6.2.3.1. Resistors in series: Rseries = R1 + R2 + R3 + …
3.1.6.2.3.2. Resistors in parallel: 1/Rparallel = 1/R1 + 1/R2 + 1/R3 + …
3.1.6.2.4. Resistively :
R = l / A where is characteristic of a given material
3.1.6.2.4.1. silver = 1.59E-8 copper = 1.72E-8 aluminum = 2.82E-8 iron = 9.7E-8
3.1.6.2.4.2. carbon = 3.5E-5
wood = 3E10 glass = 1010 to 1014
3.1.6.2.4.3. depends upon temperature(1 +
-T0) )
3.1.6.2.4.4. = 1/ = electrical conductivity of a substance
3.1.6.2.5. Power Loss P = IV = I2R
3.1.6.3. Advanced
3.1.6.3.1. Electric current I = dQ/dt = n q v A
3.1.6.3.2. Electric current density j = I / A = n q v
3.1.6.3.3. Ohms law with current density j = E where = conductivity
28
3.1.7.Direct Electrical Currents <CJ chap 20.8-20.15 >
3.1.7.1. Discussion
3.1.7.1.1. Kirchhoff’s Laws:
3.1.7.1.1.1. Sum of currents entering a junction must equal the sum leaving the junction (node)
3.1.7.1.1.2. Sum of voltages across each element in any closed loop must be zero.
3.1.7.1.2. Discuss:
3.1.7.1.2.1. Voltmeter
3.1.7.1.2.2. Galvanometer
3.1.7.1.2.3. Ammeter
3.1.7.1.3. Discuss household wiring 110V and 220V, circuit breakers, …
3.1.7.2. Mathematical
3.1.7.2.1. RCV circuit : = RC is the time constant of the circuit
3.1.7.2.1.1. If charging from a voltage V applied at t=0 then q(t) = Q0(1-e-t/RC) and i(t) = (V/R)e-t/RC
3.1.7.2.1.1.1. where Q0 = CV
3.1.7.2.1.2. If discharging a charged capacitor from t=0 then q(t) = Q0e-t/RC and i(t) = I0e-t/RC
3.1.7.2.1.2.1. where Q0 = initial charge on the capacitor, and I0 = Q/RC
3.1.7.3. Advanced
3.1.7.3.1. Solve the differential equations for the RCV circuit to derive the equations above
3.1.7.3.2. Practice with complex circuit diagrams
3.1.7.3.3. Take the resistance, r, of the battery into account in circuits
3.1.7.3.4. Discuss the use of the Wheatstone bridge to measure an unknown resistor
29
3.2. Magnetism
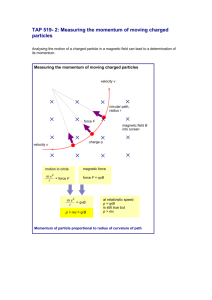
3.2.1.Magnetic Fields <CJ chap 21.1-21.6 >
3.2.1.1. Discussion
3.2.1.1.1. General discussion of magnetism, N & S poles, magnetic field lines, earths magnetic field, direction N
3.2.1.1.2. Motion of a charged particle in a magnetic field – Right Hand Rule
3.2.1.1.2.1. Cosmic rays to earth go to poles thus protecting the earth from this radiation
3.2.1.1.3. The units of the magnetic field are the Tesla = Nt/(C m/s). One Tesla is a very intense magnetic field
3.2.1.1.3.1. The Gauss is defined by 1 T = 104 Gauss. The earth’s magnetic field is about ½ Gauss.
3.2.1.1.4. This force on a current segment in a magnetic field opens up the possibility of the motor
3.2.1.2. Mathematical
3.2.1.2.1. Magnetic Force on a moving charge is F = q v x B = q v B sin
3.2.1.2.2. Magnetic force on a current segment F = I r x B
3.2.1.2.3. Magnetic dipole moment defined: = I A where I = current in a loop of area A
3.2.1.2.3.1. Torque on a magnetic dipole in a magnetic field B is = x B
3.2.1.2.3.2. The potential energy of a magnetic dipole in a magnetic field is U = - B
3.2.1.2.4. Radius & Period of the path of a charged particle in a magnetic field r = mv/qB T=2m/qB
3.2.1.3. Advanced
3.2.1.3.1. Magnetic force on a current segment dF = I dr x B where I = the current in amps
3.2.1.3.1.1. Derivation: dF = dq (dr/dt) x B then divide dq by dt to get current I leaving dr
30
3.2.2.Magnetic Field Sources <CJ chap 21.7-21.10 >
3.2.2.1. Discussion
3.2.2.1.1. The Right Hand Rule (RHR): determines the x product, and the direction of B around currents
3.2.2.1.2. Magnetism in matter arises from the currents in matter and magnetic moments of particles
3.2.2.1.2.1. The magnetic moment of a loop of current is the ‘fundamental magnet’
3.2.2.1.2.2. Use the RHR to get the N and S poles for such a loop.
3.2.2.1.3.
3.2.2.2. Mathematical
3.2.2.2.1. Biot-Savart law: Magnetic fields arise from the motion of electric charge, i.e. electric currents
3.2.2.2.1.1. B = (o/4) I s x runit / r2 where I = current, s = length of wire, B = mag. Field
3.2.2.2.1.2. (o/4) = km = 1E-7 exactly thus defining the value of o, the permeability of free space
3.2.2.2.1.3. The unit vector runit points from the current segment s to the point r where B is to be found
3.2.2.2.2. B = o I /(2a) gives the magnetic field a distance ‘a’ from an infinite straight wire
3.2.2.2.3. B = o I R2 /(2 x2 + R2)3/2 = B field on the axis a distance x from a circular loop of current I, Radius R,
3.2.2.2.4. F/s = o I1 I2 /(2a) = force between two long parallel wires a distance ‘a’ apart with currents I 1 and I2
3.2.2.2.4.1. Defines the Ampere if the force per m = 2E-7 results from equal currents I1 and I2 of both 1 Amp
3.2.2.2.5. Ampere’s law: B x distance around a closed loop = oI
3.2.2.2.6.
B = o n I = B field in a solenoid with n = N / l (# of turns per length)
3.2.2.3. Advanced
3.2.2.3.1. Biot-Savart law: dB = (o/4) I ds x runit / r2 where I = current, ds = length of wire, B = mag. Field
3.2.2.3.2.
Gauss Law for Magnetism
3.2.2.3.3.
Ampere’s law:
3.2.2.3.4.
Ampere’s law modified by Maxwell displacement current
B
B
d = 0 = the magnetic flux through any closed surface
ds =o I
B
ds =o I + o o d(
E d)/dt
3.2.2.3.4.1. Using a cylindrical surface around a wire ending in a capacitor then EA = Q/o
3.2.2.3.4.2. thus o d/dt = dQ/dt = IMaxwell & using this IMaxwell in addition to the I in Amperes law gives result
3.2.2.3.5. The Magnetization vector, M, = magnetic moment per unit volume and
3.2.2.3.5.1. Thus B = B0 + Bm = B0 + oM = o (H + M)
3.2.2.3.5.2. For paramagnetic and diamagnetic substances, M = H where = the magnetic susceptibility
3.2.2.3.5.2.1.
with m = o (1 + ) substances are classified as
3.2.2.3.5.2.2.
paramagnetic m > 0 , diamagnetic m < 0 , and ferromagnetic m >> 0
31
3.2.3. Faraday’s Law <CJ chap 22.1-22.6 >
3.2.3.1. Discussion
3.2.3.1.1. Faraday’s discovery of induction allows for the creation of a voltage by moving a loop in a B field
3.2.3.1.1.1. Either the flux can change due to the motion or orientation of the wire or loop or
3.2.3.1.1.2. The flux can change due to a changing magnetic field or even the motion of the source magnet
3.2.3.2. Mathematical
3.2.3.2.1. Faraday’s law of induction: V = - /t and = B A the magnetic flux through an open surface
3.2.3.2.2. Lenz’s law: the induced EMF will create a magnetic flux to oppose the change in magnetic flux
3.2.3.2.3. EMF from the motion of a conductor in a B field: V = -B s v for a conductor of length s moving at v
3.2.3.3. Advanced
3.2.3.3.1.
Faradays law V = - d/dt and =
But V (induced emf) around a closed circuit is V =
32
B d the magnetic flux through an open surface
E ds = - d/dt
B d
3.2.4.Induction <CJ chap 22.7-22.10 >
3.2.4.1. Discussion
3.2.4.1.1. Induction also allows for the concept of a transformer which can increase or decrease AC voltage
3.2.4.2. Mathematical
3.2.4.2.1. Self-Inductance: the induced voltage is VL = - N ddt = - L dI/dt
3.2.4.2.1.1. The unit of inductance is the Henry (H)
3.2.4.2.2. The equation for a transformer is V1 / N1 = V2 / N2
3.2.4.2.2.1. Since the transformer power input must equal power output we also have V1I1 = V2I2
3.2.4.2.3. RLV Circuits: I(t) = (V/R) (1-e-t/) where = L/R is the time constant of the RL circuit
3.2.4.2.4. Energy stored in the magnetic field: U = ½ L I2
3.2.4.3. Advanced
3.2.4.3.1. Solve the RLV circuit: V –RI –L dI/dt =0 which is an inhomogeneous first order differential equation
33
3.2.5.Alternating Electric Currents <CJ chap 23 >
3.2.5.1. Discussion
3.2.5.2. Mathematical
3.2.5.3. Advanced
3.2.5.3.1. Solve the general RCLV circuit: L d2q/dt2 + R dq/dt + (1/C) q = V0 Use q(t) = q0 et Find
3.2.5.3.2.
Define = -R/2L 02 = 1/LC then = -
3.2.5.3.3.
Three cases result from the square root:
3.2.5.3.3.1. Over damped then q(t) = A et-
2 o 2
2 o 2 t
+ B et-
2 o 2 t
3.2.5.3.3.2. Critically damped then q(t) = A e t + B t e t (degenerate case)
3.2.5.3.3.3. Underdamped
then q(t) = A e t+1t + B e t-1t where 2 = 2 - 2
34
3.3. Electromagnetism
3.3.1.Maxwell’s Equations <CJ chap 24.1-24.3 >
3.3.1.1. Discussion
3.3.1.2. Mathematical
3.3.1.3. Advanced
3.3.1.3.1. Lorentz force equation:
3.3.1.3.2. Maxwell’s Equations
F=qE+ qvxB
3.3.1.3.2.1. Gauss’ law of electricity
( = dp /dt by Newton’s equation of motion)
dqinside /0
B
or
E / o where is the charge
density
3.3.1.3.2.2. Gauss’ law of magnetism
3.3.1.3.2.3. Faraday’s law of induction
E ds = - d/dt
3.3.1.3.2.4. Ampere’s law modified by Maxwell
B o j o o
d = 0
B
B0
or
B dor E
ds =o I + o o d/dt (
dE
where j is the current density
dt
3.3.1.3.2.5. The differential forms use the following two equations:
3.3.1.3.2.5.1.
Gauss ' Theorem : A d Ad 3 x
S
3.3.1.3.2.5.2.
Greens Theorem:
A ds ( A) d
C
35
and
dB
dt
E d) or
3.3.2.Solution in a Vacuum – EM Waves <CJ chap 24.4-24.7 >
3.3.2.1. Discussion
3.3.2.1.1. Maxwell solved his equations in a vacuum – meaning no charges or currents on the RHS
3.3.2.1.2. He found solutions:
3.3.2.1.2.1. With oscillating E & B perpendicular fields at any frequency, and any amplitude such that E = cB
3.3.2.1.2.2. The oscillations move at exactly the speed of light, c = (0 0)-1\2 with E & B perpendicular to c
3.3.2.1.3. The waves carry both energy and momenta and are transverse with the E direction giving polarization
3.3.2.1.3.1. Polarization can also be circular (left or right handed) corresponding to the spin of the photon
3.3.2.1.4. The Doppler effect applies to EM waves (like to sound) and raises frequencies of oncoming waves
3.3.2.2. Mathematical
3.3.2.2.1. The wave is given by E(x,t) = E0 cos (t + kx + ) where is the phase in radians and
3.3.2.2.1.1. The angular frequency is the angular velocity & related to the period T (=1/f) by T = 2
3.3.2.2.1.2. The wave number k is related to the wave length of a full wave by k = 2
3.3.2.2.1.3. And E0 is the amplitude of the wave restricted to E0 = c B0
3.3.2.2.1.4. Likewise, B(x,t) = B0 cos (t + kx + ) with the same values and such that f = /k = c
3.3.2.2.2. Energy and momenta densities of the wave:
3.3.2.2.2.1. The energy density is given generally by u = (½)e0 E2 + (1/(20 )) B2
3.3.2.2.2.1.1. but one must insert the root mean square value for the oscillating fields as Erms = E0/(2)1/2
3.3.2.2.2.1.2. and likewise for the B field
3.3.2.2.2.1.3. The energy and momenta are equally distributed in the E and B fields.
3.3.2.2.2.2. The intensity of the EM wave is the power/m2 = S = c u where u is the energy density above
3.3.2.2.3. Doppler effect is given when vrel << c by fo = fs (1 vrel/c) where refers to approach or recede
3.3.2.3. Advanced
36
4. Light & Optics
4.1.1. Reflection of Light & Mirrors <CJ chap 25 >
4.1.1.1. Discussion
4.1.1.1.1. Flat Mirrors
4.1.1.1.1.1. Law of reflection is that the angle (to the normal) of reflection equals the angle of incidence
4.1.1.1.1.2. The left and right handiness is reversed in a mirror (eg with handwriting)
4.1.1.1.1.3. A reflected image is as far behind a mirror as the object is in front, and is upright
4.1.1.1.2.
Spherical Mirrors
4.1.1.1.2.1. Using a normal to the surface, one can show that the focal length is half of the radius of the mirror
4.1.1.1.2.2. The focal length is here defined as the position of a focused image from infinity
4.1.1.1.2.3. Likewise for a a reflection in either a convex or concave mirror focal length is half the radius
4.1.1.1.2.4. Note that not all rays from infinity focus exactly there but only those near the center
4.1.1.1.2.5. Note ray tracing to form an image of an object in convex & concave mirrors (Example)
4.1.1.1.2.6. A concave mirror gives enlarged, upright, virtual images in front of the mirror
4.1.1.1.2.7. A convex mirror gives a smaller, upright, virtual image behind the mirror
4.1.1.2. Mathematical
4.1.1.2.1. Law of reflection is that the angle of incidence equals the angle of reflection i=r
4.1.1.2.2. The focal length of both convex and concave mirrors is given by f = R/2 where R is the radius
4.1.1.2.3. Let do and di be the distances of the object and image to the mirror then 1/do + 1/di = 1/f
4.1.1.2.4. And the magnification is m = - di /do (if negative then image is inverted, if positive then upright)
4.1.1.3. Advanced
37
4.1.2. Refraction of Light & Lenses <CJ chap 26 >
4.1.2.1. Discussion
4.1.2.1.1. The Index of Refraction is ratio of the speed of light in vacuum to the speed in the material
4.1.2.1.1.1. Examples are diamond 2.419, Crown glass 1.523, Benzene 1.501, Water 1.333, Air 1.000293
4.1.2.1.1.2. Refraction of different substances gives prism effect to lead crystal etc
4.1.2.1.2. Total internal reflection – view from beneath water – how a fish sees the fisherman
4.1.2.1.3. Total internal reflection used in fiber optics and prisms for binoculars (glass has index of ref = 42 deg)
4.1.2.1.4. Brewster’s angle: the angle for a substance that polarizes the reflected light with reflect=refract
4.1.2.1.5. Dispersion of light:
4.1.2.1.5.1. Prisms – note red is least diverted (and on the pointed side of prism)
4.1.2.1.5.2. Rainbows: sunlight enters and is internally reflected in water drops: red is bent least (rainbow top)
4.1.2.1.6. Farsightedness (hyperopic) (use converging lens) and nearsightedness (myopic) (use diverging lens)
4.1.2.1.7. Lenses in combination (see diagrams)
4.1.2.1.8. Lens aberrations: spherical and chromatic aberration
4.1.2.2. Mathematical
4.1.2.2.1. Refraction:
4.1.2.2.1.1. Index of refraction n = c/v where v is the speed of light in the material (always n >= 1)
4.1.2.2.1.2. Snell’s law of refraction n1 sin 1 = n2 sin 2 (light passing from media 1 to 2 angles rel. to normal)
4.1.2.2.2. Total internal reflection
4.1.2.2.2.1. Use Snell’s law with 2 = 90 deg. To get c = sin-1(n2 /n1)
4.1.2.2.3. Brewster’s Law: reflect=refract occurs when tan B = n2 /n1 and the reflected light is polarized
4.1.2.2.4. Lenses
4.1.2.2.4.1. Converging lens formula 1/do + 1/di = 1/f with magnification m = hi /ho = - di /do
4.1.2.2.4.2. Sign conventions:
4.1.2.2.4.2.1. f is + for converging lens, - for diverging lense
4.1.2.2.4.2.2. do is + if object is to the left of the lens (real object) and – if to the right (virtual object)
4.1.2.2.4.2.3. di is + for a (real) image formed to the right of the lens by real object, and – to the left
4.1.2.2.4.2.4. m is + for an image that is upright with respect to the object, and – for inverted
4.1.2.2.4.3. Magnifying glass magnification m approx.= (1/f – 1/di) N where N = dist. of near point to eye
4.1.2.2.4.4. Telescope m approx.=-fo/fe where fo & fe are the focal lengths of the objective and eyepiece lens
4.1.2.2.4.5. Microscope m approx.= -(L-fe)N/ (fofe) where L is the dist. between the lenses & N is near point
4.1.2.3. Advanced
38
4.1.3. Interference & Wave Nature of Light <CJ chap 27 >
4.1.3.1. Discussion
4.1.3.1.1. Principle of linear superposition: resultant disturbance is the sum of individual disturbances
4.1.3.1.2. Interference is constructive if waves are in phase, destructive if out of phase
4.1.3.1.3. Thin film interference described as with gasoline on water
4.1.3.1.4. Diffraction through a slit: resolving power is when the first dark band falls on the central bright band
4.1.3.1.5. Diffraction grating – used to diffract light and create a spectroscope
4.1.3.2. Mathematical
4.1.3.2.1. Young’s double slit experiment: sin = m(d) constructive with m = 0, 1, 2; destructive m = 1/2 , 3/2..
4.1.3.2.2. Thin film film = vacuum /n and
4.1.3.2.2.1. thus difference of distance = 2thickness + ½ film (due to reflection) =½ film , 3/2 film…
4.1.3.2.2.2. then subtracting ½ film from each side one gets 2 t = 0, 1film , 2film , 3film …
4.1.3.2.2.3. then solving for t one gets t = m film /2 where m = 0, 1, 2, 3, …
4.1.3.2.3. Diffraction through a single slit gives: sin = m /W where m = 1, 2, …, W=width, for destructive
interference
4.1.3.2.3.1. min = 1.22 /D for the minimum resolution between two objects using an aperture D
4.1.3.2.3.2. Diffraction grating maxima are sin = m /d m = 1, 2, 3 where d is the slit separation
4.1.3.2.3.2.1. red is dispersed by the greatest angle and violet the least
4.1.3.3. Advanced
39
5. Relativity
5.1.1. Special Relativity <CJ chap 28 >
5.1.1.1. Discussion
5.1.1.1.1. Constancy of c, the velocity of light, to all observers presents a conflict between Newton & Maxwell
5.1.1.1.1.1. Maxwell EM equations predict that c = (00)-1/2 = 3E8 m/s in vacuum to all frames & observers
5.1.1.1.1.1.1. Michelson & Morley proved this was true using the earths motion: Explain
5.1.1.1.1.1.2. Attempts to explain c=const. with ‘ether’ theories etc were flawed.
5.1.1.1.1.2. Newtonian space time is related by x’=x-Vt & t’=t which implies v’ = v – V ie velocities add
5.1.1.1.1.2.1. This is confirmed by our intuition and everyday experience – Examples of cars:
5.1.1.1.2. Einstein assumed three postulates and allowed for a more general relationship for x & t
5.1.1.1.2.1. Assumption 1: The laws of physics are identical in inertially related (constant v) frames
5.1.1.1.2.2. Assumption 2: The speed of light in vacuum is a constant.
5.1.1.1.2.3. Assumption 3: The relationship between x & t in two frames is linear for the 4 dimensions
5.1.1.1.3. Einstein showed that space (length) and time are not each invariant but transform as a 4 dim. vector
5.1.1.1.3.1. This 4-vector of space-time described an event for one observer & related it to another observer
5.1.1.2. Mathematical
5.1.1.2.1. Lorentz Contraction: One can then show that length is contracted by L = L0 (1-v2/c2)1/2
5.1.1.2.1.1. where L is the observed length and L0 is the length in its own rest frame
5.1.1.2.2. Time Dilation: One can also show that time is expanded by t = t0 /(1-v2/c2)1/2
5.1.1.2.2.1. where t is the observed length and t0 is the length in its own rest frame
5.1.1.2.2.2. These effects are only about 1% when one gets to a tenth of the speed of light: v/c =1/10
5.1.1.2.2.3. Below that relativity is essentially negligible. Yet effects explode near v=c.
5.1.1.2.3. The old formula for KE = p2/(2m) is now replaced by: (E/c)2 - Px,2 - Py2 - Pz2 = m2c2 = E2/c2 - P2
5.1.1.2.3.1. Now using E2/c2 - P2 = m2c2 to solve for E we get
5.1.1.2.3.2. E c p m c and when p 0, E mc which is the famous Einstein equation
2
2
2 4
2
5.1.1.2.4. In relativity neither mass nor energy is separately conserved but only their combination via E=mc2
5.1.1.2.5. The negative sign was ignored for 20 years until it was shown to correspond to ‘antimatter’
5.1.1.2.5.1. Antimatter is identical to matter except of opposite charge and it annihilates corresponding matter
5.1.1.2.6.
Next we solve E2/c2 - P2 = m2c2 for m (choose units with c=1):
m E 2 p 2 giving 3 cases:
5.1.1.2.6.1. E>p giving m >0 and v<c This is ordinary matter and must move slower than c
5.1.1.2.6.2. E=p giving m = 0 and v=c These massless particles, such as photons, always have v=c
5.1.1.2.6.3. E<p giving m imaginary and thus v>c are called tachyons and must move faster than light
5.1.1.2.6.4. Imaginary mass particles (tachyons) have never been observed nor has negative mass
5.1.1.2.6.5.
5.1.1.3. Advanced
5.1.1.3.1. The Lorentz transformation derived: x’ = L x where x = (ct, x, y, z) = (x0, x1, x2, x3) = x
5.1.1.3.1.1. This set of four ‘coordinates’ of an event, is a 4 dimensional vector under L
5.1.1.3.1.2. A sphere of light, ct=r must be seen the same by all observers thus c 2t2-r2 = invariant
5.1.1.3.1.3. Compute this in two dimensions to get (x’0, x’1) = (L00, L01,/ L10, L11) (x0, x1) then
5.1.1.3.1.4. One obtains (L00, L01,/ L10, L11) = (ch, sh / sh, ch ) where th= v/c
5.1.1.3.1.4.1. because of ch2 sh2 = 1 (compare to cos2 + sin2 = 1)
5.1.1.3.1.4.2.
ch
1
1
2
v
c2
, sh
v/c
1
v2
c2
5.1.1.3.2. The scalar product, defining the metric properties of the space is A B = g AB where
5.1.1.3.3. The metric for this invariant is gis defined by g = (+1, -1,-1,-1) and g off diagonal
5.1.1.3.4. Thus d2 = gdx dx is invariant and is called the proper time: d2 = c2 dt - dr2
5.1.1.3.4.1.1. because it gives the invariant time interval on a clock on the particle that is moving
5.1.1.3.4.2. As time is part of a 4 vector, we cannot effectively use it to take derivatives and must use
5.1.1.3.4.2.1. d thus giving a 4-vector velocity of v = c dx /d (note that ‘c’ give it dimensions of vel)
5.1.1.3.4.2.2. and one can verify that the invariant length of this vector is always c : gvvc2
5.1.1.3.5. The 4-vector momentum is thus defined as mass times velocity: p = m v then gppm2c2
5.1.1.3.5.1. Thus energy & momentum form a 4 vector: (E/c, Px, Py, Pz) =P and transform like dx
5.1.1.3.6. When gppm2c2 is written out it becomes: (E/c)2 - Px,2 - Py2 - Pz2 = m2c2 = E2/c2 - P2
5.1.1.3.6.1. This is the relativistic equation relating energy, momentum and mass that replaces E= p 2/(2m)
5.1.1.3.6.2. Now using E2/c2 - P2 = m2c2 to solve for E we get
5.1.1.3.6.2.1.
E c 2 p 2 m2 c 4 and when p 0, E mc 2 which is the famous Einstein equation
40
5.1.2. General Relativity & Astrophysics <CJ chap 28.8 & not in text >
5.1.2.1. Discussion
5.1.2.1.1. Special relativity addresses observers moving with relative constant velocity only
5.1.2.1.2. General relativity deals with cases where one observer is accelerated relative to the other
5.1.2.1.3. Rotating platform: Einstein argued that a rotating platform gives a non-Euclidian (curved) geometry
5.1.2.1.3.1. As one moves outward, the Lorentz contraction shortens circumferences to ever smaller values
5.1.2.1.3.2. Also as one moves outward, clocks slow down because of time dilation
5.1.2.1.3.3. Far from the center, where v is almost equal to c, the circumference is near 0 & time stands still
5.1.2.1.3.4. So space and time in accelerated frames is unquestionably curved (not ‘flat’)
5.1.2.1.4. Elevator experiment: Einstein compared an accelerated elevator to the same one in gravity with a=g
5.1.2.1.4.1. No experiment with regular matter would distinguish g from a as all mass has the same g
5.1.2.1.4.2. Yet light is not bent by gravity (as per Newtons equation) but light ‘appears’ bent with acceleration
5.1.2.1.4.3. Einstein argued that by symmetry, light should be bent the same amount by g as by a
5.1.2.1.4.4. This violates the Newton formula for gravity as light has a mass of zero
5.1.2.1.4.5. His prediction that light from a distant star is bent by the sun was verified
5.1.2.1.5. Gravity (and acceleration) is thus seen as a warped space time where masses follow geodesics
5.1.2.1.6. The integration of Einstein’s theory is still not reconciled with modern theories of other forces
5.1.2.2. Mathematical
5.1.2.2.1. A rotating platform circumference is shortened by the Lorentz contraction: C = C0 (1-v2/c2)1/2
5.1.2.2.1.1. and one can compute at what point the circumference begins to get smaller and at v=c is zero
5.1.2.2.2. At larger distances from the center, time dilation effects slow time by t = t 0 /(1-v2/c2)1/2
5.1.2.2.2.1. where t is the observed length and t0 is the length in its own rest frame
5.1.2.2.3. In both equations, v = r where is the angular velocity of the platform
5.1.2.3. Advanced
5.1.2.3.1. The mathematical theory of curved spaces is called Riemannian geometry or differential geometry
5.1.2.3.2. The fundamental concept is the metric gwhich is used to define scalar products thus length & angle
5.1.2.3.3. Particles (as well as light) follow the shortest distances (called geodesics) in such curved spaces
5.1.2.3.4. Einstein’s theory thus relates gfor the space to T which is the energy momentum tensor density
41
6. Quantum Theory – Atomic, Nuclear, & Particle Physics
6.1.1. Foundations of Quantum Mechanics – Particles & Waves <CJ chap 29 >
6.1.1.1. Discussion
6.1.1.1.1. Cavity radiation refers to EM radiation from a hole inside a substance -also called blackbody radiation
6.1.1.1.1.1. Is dependent upon the temperature and independent of the substance making the cavity
6.1.1.1.1.2. Cavity radiation was found to have a wavelength spectra that could not be explained by theory
6.1.1.1.1.3. Planck (1900) proposed that the walls consist of oscillators that emit & absorb only certain quanta
6.1.1.1.1.4. where Eem = n h f where n = 1,2,.. f = the frequency of radiation, and h is a constant 6.626E-34
6.1.1.1.2. Photoelectric effect is the emission of electrons from a metal when radiated by ultraviolet light
6.1.1.1.2.1. Problem 1: The energy of the electrons is independent of the light intensity but depends only on f
6.1.1.1.2.2. Problem 2: Below a given f of light, no electrons are emitted no matter how intense the light is
6.1.1.1.2.3. Problem 3: The effect of emission is immediate no matter how low the intensity
6.1.1.1.2.4. These problems were counter to the Maxwell theory of EM radiation as was cavity radiation
6.1.1.1.3. Einstein explained both phenomena and founded quantum theory postulating photons that Eem =hf
6.1.1.1.3.1. Thus light consisted of these ‘quanta’ of pure massless energy also with momenta P=h/
6.1.1.1.3.2. Thus the view of EM radiation as oscillating E and B fields is only an approximation to photons
6.1.1.1.4. Arthur Compton in 1923 scattered photons from electrons and showed that ’- = (h/mc)(1-cos)
6.1.1.1.4.1. This confirmed the Einstein photon hypothesis experimentally
6.1.1.1.5. Louis De Broglie in 1923 proposed that the same photon equations Eem =hf, p=h/ apply to matter
6.1.1.1.5.1. Thus given a particles energy E and momentum p, one can compute an associated f &
6.1.1.1.5.2. In 1927, Davisson & Germer & Thompson confirmed wave interference effects scattering e 6.1.1.1.5.3. This scattering of e- from a crystal gave interference patterns only possible for a wave like X rays
6.1.1.1.6. In 1925, Erwin Schrödinger proposed his equation for the ‘motion’ of this ‘matter wave’ (x,y,z,t)
6.1.1.1.7. In 1925 Werner Heisenberg also proposed an alternate formulation for in terms of matrix theory
6.1.1.1.8. In 1926 P.A.M. Dirac presented a unifying mathematical theory that showed these theories equivalent
6.1.1.1.9. Heisenberg later showed that contains information on both the particles position and momenta BUT
6.1.1.1.9.1. to know more about the position one looses knowledge of the momenta and conversely as:
6.1.1.1.9.2. Heisenberg uncertainty principle gives the product of these uncertainties as x p >= h/4
6.1.1.1.9.3. Also one has an equivalent equation for energy and time: t E >= h/4
6.1.1.1.9.4. Heisenberg’s uncertainty principle has deep implications for what is simultaneously knowable
6.1.1.2. Mathematical
6.1.1.2.1. A particle of mass m, in a box of length L must have an integer number of half waves
6.1.1.2.1.1. Thus n / 2 = L thus = 2 L / n thus pn = h/ = n h / 2L resulting in a discrete set of momenta
6.1.1.2.1.2. Using E = P2/(2m) we get En = n2 h2 /(8m L2) giving the discrete energies of a particle in a box
6.1.1.3. Advanced
42
6.1.2. Atomic Theory <CJ chap 30 >
6.1.2.1. Discussion
6.1.2.1.1. The Thompson model of the atom held that positive charge was spread out like a pudding.
6.1.2.1.2. In 1911 Rutherford scattered particles from gold foil and showed the nuclear size was ~1E-15m
6.1.2.1.3. This raised the problem of why the electron did not spiral into the center with infinite radiation
6.1.2.1.4. Atomic spectra was observed at discrete frequencies rather than continuous emissions
6.1.2.1.4.1. This implied discrete orbits for the electron but what equations would make this work
6.1.2.1.5. In 1913 Bohr proposed his model of the atom with quantized orbits and discrete transitions
6.1.2.1.6. The Bohr model assumes that angular momentum is quantized.
6.1.2.1.7. The Pauli exclusion principle prevents two electrons from being in the same shell simultaneously
6.1.2.1.8. Einstein predicted that if an excited atom is hit with a photon of the decaying energy then ..
6.1.2.1.8.1. rather than being absorbed, the photon will stimulate the emission of another photon in phase
6.1.2.1.8.2. This principle is the basis for the operation of a laser
6.1.2.1.8.3. LASER means Light Amplification by Stimulated Emission of Radiation
6.1.2.1.9. X Rays were discovered by Wilhelm Roentgen by hitting electrons on a metal target
6.1.2.2. Mathematical
6.1.2.2.1. Atomic spectra was observed to obey: 1/= R(1/n12 – 1/n22) with terminology of:
6.1.2.2.1.1. n1 = 1 Lyman series , n1 = 2, Balmer series, n1 = 3 Paschen series …
6.1.2.2.1.2. Bohr’s model of quantized orbits assumed a quantized angular momentum of Ln=n h/(2), n= 1,2
6.1.2.2.1.2.1. This assumption in addition to the classical equations gave workable orbits:
6.1.2.2.1.2.2. One balances Coulomb force with centripetal force: mv2/r = kZe2/r where Z=# protons
6.1.2.2.1.2.3. Using these two equations, the radius must be rn = h2 n2 / (42kme2Z) =5.29E-11 n2/Z
6.1.2.2.1.2.4. The electron’s energy is KE+PE = E = (1/2) mv2 –kZe2/r
6.1.2.2.1.2.5.
Thus En = 22mk2e4/h2)(Z2/n2) = -13.6 eV Z2/n2 = -2.18E-18 J Z2/n2
6.1.2.2.1.2.5.1. Note that the factor 13.6 eV is the ionization energy of hydrogen (Z=1 & n=1)
6.1.2.2.1.2.6. Since 1/f/c = E/hc then 1/22mk2e4/(ch3) (Z2/n2)
6.1.2.2.1.3. De Broglie: If the electron ‘wave’ had to meet constructively with itself then Cir. = 2r = n n h/p
6.1.2.2.1.3.1. Consequently we get quantized angular momentum as r p = L = n (h/ 2)
6.1.2.2.2. The Schrödinger equation solution to the hydrogen atom gives the following energy levels:
6.1.2.2.2.1. The principle quantum number, n = 1, 2, 3, …..
6.1.2.2.2.1.1. The principle quantum numbers 1, 2, 3,..are denoted by the shell names: K, L, M
6.1.2.2.2.2. The orbital angular momentum l has the values 0, 1, 2, 3, … (n-1) where L = ((l( l+1))1/2)h/2
6.1.2.2.2.2.1. The orbital angular quantum numbers 0, 1, 2, ..are denoted by the letters s, p, d, f, g, h,
6.1.2.2.2.3. There is also a ‘magnetic quantum number’ that has the values – l, - l+1, … l-1, l
6.1.2.2.2.3.1. The magnetic quantum number was seen when levels were split with a magnetic field
6.1.2.2.2.3.2. It is known to correspond to the z component of the angular momentum Lz
6.1.2.2.2.4. A final splitting of the energy levels occurred due to the z component of the spin of the electron
6.1.2.2.2.5. The associated counting of levels now exactly counts for the number of electrons in each orbit
6.1.2.2.2.5.1. The maximum number of electrons in a shell are 2(2 l+1)
6.1.2.2.2.5.2. The denotation of electrons in a shell is say: 2p5 thus n=2, l =1, and with 5 electrons
6.1.2.2.2.5.3. Thus the configuration of Carbon (6 electrons) is 1s2 2s2 2p2
6.1.2.2.3. Pauli Exclusion Principle: No two identical fermions can occupy the same state at the same time
6.1.2.2.3.1. A Fermion is an elementary particle with a spin of ½, 3/2, 5/2, 7/2, … times h/(2)
6.1.2.2.3.1.1. Electrons, protons, neutrons, neutrinos, muons, … are all Fermions
6.1.2.2.3.2. A Boson is an elementary particle with a spin of 0, 1, 2, 3, … times h/(2) e.g. a photon, pion…
6.1.2.2.3.2.1. Bosons actually ‘prefer’ to be in the same state rather than being prevented
6.1.2.2.3.3. Without the exclusion principle, all electrons would go to the atoms lowest state & not fill shells
6.1.2.2.3.3.1. Then without a tendency to fill shells, there would be no chemical bonding, & no biology
6.1.2.2.3.3.2.
6.1.2.3. Advanced
43
6.1.3. Nuclear Theory & Radioactivity
<CJ chap 31 >
6.1.3.1. Discussion ( h )
6.1.3.1.1. Nucleons are protons or neutrons – the particles that make up the nucleus of the atom
6.1.3.1.1.1. The neutron was discovered in 1932 by Chadwick with a mass slightly larger than the proton
6.1.3.1.1.2. The atomic number, Z =the number of protons, and A the mass number = the number of nucleons
6.1.3.1.1.2.1.
A nucleus is written as
A
Z
X where X is the chemical element corresponding to Z
6.1.3.1.1.2.2. Isotopes are nuclei with the same number of protons but differing numbers of neutrons
6.1.3.1.1.2.3. The nuclear forces felt by both the p and n are essentially identical
6.1.3.1.1.2.4. The binding energy is the amount of energy needed to separate the nucleons
6.1.3.1.1.2.5. The mass defect is the binding energy expressed in mass equivalence via E = mc2
6.1.3.1.1.2.6. The binding energy per nucleon is greatest in mid-range of A (Fe) and less in Li and U
6.1.3.1.1.3. Nuclear reactions:
6.1.3.1.1.3.1. Rutherford (1919) observed the first ‘transmutation of an element’ with + N -> O + H
6.1.3.1.1.4. Radioactivity is the decay or disintegration of an unstable nucleus
6.1.3.1.1.4.1. decay: The emission of an alpha particle or He nucleus (2p+2n) – easy to stop
6.1.3.1.1.4.1.1. Example of decay
6.1.3.1.1.4.2.
238
92
U ->
234
90
Th + 42 He + 4.3 MeV of energy
decay: The emission of an electron (or positron) via n -> p + e- + e - not hard to stop
6.1.3.1.1.4.2.1. Example of decay
234
90
Th ->
234
91
Pa +
0
1
e e
6.1.3.1.1.4.3.
6.1.3.1.1.4.4. decay: The emission of a high energy photon releasing energy – needs lead to stop
6.1.3.1.1.4.5. n decay: The emission of a neutron directly from the nucleus
6.1.3.1.1.4.6. Half-life is the time required for half of a substance to undergo disintegration
6.1.3.1.1.4.7. Radioactive dating: Carbon 14 has a half life of 5730 years
6.1.3.1.1.4.8. The Becquerel (Bq) is the unit of radioactivity = 1 disintegration per sec
6.1.3.1.1.4.8.1. The Currie (Ci) is another unit of activity: 1 Ci = 3.70E10 Bq = 1 gr of pure radium
6.1.3.1.1.5. Biological Effects of Radiation
6.1.3.1.1.5.1. Ionizing radiation (charges particles or knocks electrons from atoms & damages cells
6.1.3.1.1.5.1.1. The SI unit of ionizing radiation is the Coulomb per kg or C/kg
6.1.3.1.1.5.1.2. The Roentgen (R) = 2.58E-4 C/kg is a more common historical unit
6.1.3.1.1.5.2. Yet this measures only the ionization effect and not the effect on tissue for which we use:
6.1.3.1.1.5.2.1. Absorbed Dose = (Energy absorbed) / (Mass absorbing) unit = Grey (Gy)=J/kg
6.1.3.1.1.5.2.2. Radiation Absorbed Dose (RAD) = 0.01 Gy is another common unit
6.1.3.1.1.5.3. To compare the damage of absorbing different kinds of radiation we define:
6.1.3.1.1.5.3.1. Relative Biological Effectiveness (RBE) = (Dose of 200KeV X-rays Effect) / (Dose )
6.1.3.1.1.5.3.2. Then Biologically Equivalent Dose (rems) = Absorbed Dose (in rads) x RBE
6.1.3.1.1.5.3.2.1.
rem stands for roentgen equivalent man
6.1.3.1.1.5.3.2.2.
Humans receive an average dose of 360 mrem/yr from all sources
6.1.3.1.1.5.3.2.3.
(cosmic rays 28, earth 28, internal 39, Radon 200, Medical/dental 43,..
6.1.3.1.1.5.3.2.4.
The general population should not get more than 500 mrem / yr
6.1.3.1.1.5.3.2.5.
Workers should not get more than 5 rem / year (eg dental assistant)
6.1.3.2. Mathematical
6.1.3.2.1. The approximate radius of the nucleus is r = 1.2E-15 A 1/3
6.1.3.2.2. Radioactive disintegration obeys N = N0 e-t thus N/N0 =1/2 = e-T1/2
6.1.3.2.3. Taking ln of both sides we get ln ½ = ln (-T1/2 ) thus T1/2 = ln2/ thus relating to T1/2
6.1.3.3. Advanced
6.1.3.3.1. Radioactive decay obeys: dN(t) = -N0 dt with the solution: N = N0 e-t
44
6.1.4. Elementary Particle Theory
6.1.4.1. Discussion
6.1.4.1.1.
<CJ chap 32 >
Nuclear fission: 0 n 92 U 92 U 56 Ba 36 Kr 3 0 n
1
235
236
141
92
1
6.1.4.1.1.1. when heavy nuclei are split into two more stable nuclei with energy release
6.1.4.1.2.
Nuclear fusion:
2
1
H 13 H 42 He 10 n
6.1.4.1.2.1. when light nuclei are combined at temperatures in the sun to make heaver ones
6.1.4.1.3. Nuclei can be plotted in two dimensions on an A vs Z plot or an N vs Z plot showing all nuclei
6.1.4.1.3.1. Either plot shows every possible nucleus and is very effective in visualizing decays
6.1.4.1.4. Elementary Particles: are classified into a number of categories, spin value, interaction strength…:
6.1.4.1.4.1. Spin: Fermions have half integer spins (½, 3/2, 5/2 ..) h , Bosons integer spins (0,1,2..) h
6.1.4.1.4.2. Strongly interacting particles are called Hadrons (participate in the nuclear or strong force)
6.1.4.1.4.2.1. Hadrons that are Fermions are called Baryons e.g. p, n, …
6.1.4.1.4.2.2. Hadrons that are Bosons are called Mesons e.g. K,
6.1.4.1.4.3. Leptons (6) are Fermions that are not Hadrons (have no strong interactions) eg e, e
6.1.4.1.4.4. Quarks (6): are the more fundamental particles that compose all of the Hadrons: u, c, s, c, b, t
6.1.4.1.4.5. Gauge particles intermediate the forces: Gravity graviton, EM , Weak Z, W , Strong gluon
6.1.4.1.5. Particles can be specified in classes by their quantum numbers (charge, strangeness, isospin, …)
6.1.4.1.5.1. Particles so plotted in these quantum number spaces have patterns as representations of groups
6.1.4.1.5.2. These group theory patterns have given a basic order to the more than 300 elementary particles
6.1.4.1.5.3. The model for this group theory is called the standard model with the following general idea:
6.1.4.1.5.3.1. All hadrons are composites made of quarks (eg p = (d+u+u), n = (d+d+u), =(d+anti u)
6.1.4.1.5.3.2. The 6 leptons and 6 quarks have very parallel interactions for EM and Weak interactions
6.1.4.1.6. Cosmology is the study of the structure and evolution of the universe
6.1.4.1.6.1. Hubble discovered that distant galaxies are all moving away from each other
6.1.4.1.6.1.1. Thus the universe is expanding, and furthermore this expansion is accelerating
6.1.4.1.6.1.2. The expansion should slow due to gravity but dark energy is causing the increase
6.1.4.1.6.1.3. The big bang is estimated to have occurred about 13.6E9 years ago
6.1.4.1.6.1.4. The cosmic background radiation is today at a temperature of about 2.7 K
6.1.4.1.6.2. There are approximately 1E11 stars in our galaxy (the Milkey Way)
6.1.4.1.6.2.1. There are approximately 1E11 galaxies in our universe
6.1.4.2. Mathematical
6.1.4.2.1. Cosmology:
6.1.4.2.1.1. Hubble’s law of expansion: v = H d where H is the Hubble parameter 0.022 m/(s ly)
6.1.4.3. Advanced
45
7. Mathematics Background <CJ chap 1 & Appendix >
7.1. The Number Systems: Originate in the acts of counting and measuring then arithmetic operations:
7.1.1.The number system: Be able to perform all + - * / ^ operations with all types:
7.1.2.Integers
7.1.2.1. Positive integers / whole numbers (counting) 1, 2, 3,… Know + - * / ab = a^b
7.1.2.2. Negative integers (inverse addition) -1, -2, -3… (from inverse addition) 3 + x =0 or x = -3
7.1.2.3. Zero – for a long time this was not a number, It was not apparent that a symbol for nothing was needed
7.1.3.Rational numbers / fractions = a/b (ratios of integers from inverse multiplication) a * x =1 or x=1/a
7.1.4.Irrational numbers / non-repeating decimals (from inverse exponentiation) ab such as (2)1/2
7.1.5.Complex numbers (also from inverse exponentiation with negative numbers) (-1)1/2 = i
7.1.5.1. imaginary numbers and complex values = a + ib
7.1.5.2. With infinity, the complex numbers close under all operations.
7.1.6.Infinity: Cantor – concept of 1 to 1 matching – multiple levels of infinity
7.1.6.1. Infinity of counting 1,2,3,… Note same value as even integers
7.1.6.1.1. Same as the infinity of rational numbers a/b
7.1.6.2. Infinity of real numbers
7.1.6.3. Infinity of functions
7.1.7. ‘Scientific notation (numbers)’: 1.23456E3 = 1.23456*103 = 1234.56 likewise 4.56E-2 = 0.0456
7.1.8. Binary numbers: 10111.0011 or even in scientific notation as 1.1001E101
7.1.8.1. Other number bases are often taken as 8 or 16 symbols.
7.1.9. ‘Uncertain numbers’ (numerical uncertainty or fuzzy numbers) 1.23 = 1.23???... Problems:
7.1.10. ‘Order of magnitude numbers’ 2E32 or maybe just 1E32 and calculations. Problems:
7.2. Data & Metadata:
7.2.1. Data is meaningless by itself except as an abstract number.
7.2.2. We generally need a form like < metadata | data > where metadata contains the units & a description.
7.2.2.1. For example < | 68.3> is simply a numerical value without metadata on its meaning
7.2.2.2. While <Jack’s mass |kg| > is metadata without a value
7.2.2.3. Then <Jacks mass|kg|68.3> is both metadata (including units) and the data.
7.2.3. Data usually takes the form of a scientific number but can also be symbolic such as e, , i,
7.3. Units – Originate as the foundational metadata as the number of basic entities (the units):
7.3.1.The value for a physical quantity is normally a quantity (real number) of fundamental ‘units’ that constitute the
quantity such as 12 ft, 4 m, 13.5 s, or 9.8 m/s2
7.3.2.The ‘fundamental’ units: length (meter = m), time (second = s), mass (kilogram = kg).
7.3.2.1. Kilogram: The mass of a specific platinum-iridium alloy cylinder in Paris.
7.3.2.1.1. The kilogram was initially defined as the mass of 10-3 M3 of water
7.3.2.2. Second: 9,192,631,700 oscillations of radiation from cesium 133 (the current definition since 1967)
7.3.2.2.1. Before 1960 was 1/86,400 of average solar day (60 s / min, 60 min/hr, 24 hr /day)
7.3.2.3. Meter: The distance light travels in 1/299,792,458 s (the current definition since 1983)
7.3.2.3.1. Originally 10-7 of the distance from the equator to the north pole. (1799)
7.3.2.3.2. Until 1960, the distance between two lines on a platinum iridium bar in Paris
7.3.2.3.3. In 1960 was defined as the 1,650,763.73 wavelengths of Krypton 86 light
7.3.3.English Units: foot, inch, hand, yard, cubit, fathom, mile, acre, (also, day, hour, min..)
7.3.4.Additional units are needed to measure: electrical current (Ampere = A), temperature (degrees Kelvin = K), and
brightness (candela = cd).
7.3.5.Dimensional analysis: only add & subtract units of the same type (apples to apples).
7.3.6. Examples of lengths, masses, times, velocity of light (3E8 m/s) & sound (1100 ft / sec)
46
7.4. Special Terms & Prefixes:
7.4.1.Prefixes:
7.4.1.1. Kilo 103, Mega 106,Giga 109, Tera 1012, Peta 1015, Exa 1018 , Zetta 1021, Yotta 1024
7.4.1.2. Milli 10-3, Micro 10-6 Nano 10-9 Pico 10-12 Femto 10-15 , Atto 10-18, Zepto 10-21, Yocto 10-24
7.4.1.3. Hecto 102, Deka 101 , Deci 10-1 , Centi 10-2,
7.4.2.The Greek alphabet – useful to know and recognize
7.4.2.1.
7.5. Supporting concepts in Logic – Origin in the special operations of logical & rational thought:
7.5.1.Special notations
7.5.1.1. There exists
7.5.1.2. Therefore
7.5.1.3. Member of
7.5.1.4. Such that
7.5.1.5. Implies
7.5.1.6. For all
7.5.1.7. Isomorphic
1-1
7.5.1.8. Infinity
7.5.1.9. Equality = and not equal
7.5.1.10.
Equal by definition or identical to
7.5.1.11.
Greater than >, less than < and also greater than or equal to >=
7.5.1.12.
Includes
7.5.1.13.
Logic & Set Theory
7.5.1.13.1. Elements 1, 0 or T, F
7.5.1.13.2. Operations AND, OR, NOT, NOR NAND, EQV, (16 operations)
7.5.1.13.3. And
7.5.1.13.4. Or
7.5.1.13.5. Not
7.5.1.13.6. Union
7.5.1.13.7. Intersection
7.5.1.13.8. Set
{s}
7.5.1.13.9. Null Set
7.6. Basic Algebra – Origin in expressing relationships among quantities represented by symbols.
7.6.1. Generally we then take the relationships and derive simpler equivalent relationships
7.6.2. Equations: Solve by doing the same thing to both sides of an equation
7.6.3. Powers add xa * xb = x(a+b) (xa)b = x(a*b)
7.6.4. Factoring x2 – y2 = (x+y)*(x-y)
7.6.5. Quadratic Equation solutions ax2 +bx +c =0 solution: x = ( b b 4ac ) / 2a
7.6.6. Linear equations: y = mx+b gives b as intersection at x=0 and m=slope
7.6.7. Simultaneous equations - solution is intersection
7.6.8. Logarithms log a + log b = log (a*b) and log a - log b = log (a/b)
7.6.8.1. y = logax implies x = ay
7.6.8.2. b loga(x) = loga(xb)
7.6.8.3. loga b = loge b / loge a this allows one to convert log from one base to another
7.6.9. Socioeconomic variables (population, electric use)
7.6.9.1. Are generally exponential in time and thus their logarithms are linear in time
7.6.9.2. Ratios of socioeconomic variables are relatively constant
7.6.9.3. Income and net worth are generally log normal (their logarithms are a normal distribution)
2
7.7. Geometry – Origin in characterizing geometrical shapes in 2 and 3 dimensions
7.7.1.Angular degrees & radians s/r
7.7.2.Area & volume
7.7.2.1. Rectangle & rectangular solids, parallelogram area
7.7.2.2. Triangle A = ½ base * height
7.7.2.3. Circle C=2 r A= r2 Sphere A = 4 r2 V = (4/3) r3
7.7.2.4. Cylinder r2 * height
47
7.8. Trigonometry – Origin is in the ratios of sides of similar triangles (which have identical angles)
7.8.1. Right triangles are the most fundamental shapes and all others can be made from these
7.8.2. Basic triangle x y r: siny/r , cos x/r , tany/x = sin / cos
7.8.2.1. The problem is then to relate these ratios (say for r = 1) to as a fraction of a circle (or better yet in radians)
7.8.3. sin2
+ cos2
review trig identities
7.8.4. Unit circle / complex numbers: eix
x
+ i sin x
z = u + iv = rei
+ i r sin
7.9.
Series expansions – Originate in solutions to equations for transcendental values
7.9.1. ex = 1 + x + x2/2! + x3/3! + x4/4! …..
7.9.2. log(1+x) = x – x2/2 + x3/3 7.9.3. sin3/3! + 5/5! and cos = 1 - 2/2! + 4/4!
7.9.4.
or sin xeix – e-ix) /2i
cos x = eix + e-ix) /2
and cos2x + sin2x = 1
7.9.5. sh(x) = sinh(x)ex – e-x)/2
ch(x) = cosh(x) = ex + e-x)/2 give the hyperbolic functions ch2x - sh2x = 1
7.9.6. Binomial series (a + b)n = an + n a(n-1)b + n(n-1) a(n-2) b2/2! + (note divide by the larger of a or b to make b small
7.9.7. Taylor series f(x) = f|(x0) + f’|(x0) (x-x0) + (1/2!) f”|(x0) (x-x0)2 …
7.10.
Calculus – Originates in the limits of ratios of infinitesimal quantities and sums of infinitesimal quantities
7.10.1. Define velocity v(t) and acceleration a(t) from position r(t). (1 dim & 3 dim)
7.10.2. Use constant a = a0 to get standard equations for esp. a = g = acceleration of gravity on earth at the surface
7.11.
Scalars, Vectors, Matrices, Tensors Linear Algebra & Matrix Theory
7.11.1. Scalar: Specified by a single real number: time, temperature, mass, volume, energy
7.11.2. Vector: An ordered n-tupe of real numbers: (x, y, z) or (x1, x2, x3) eg (1,-5,0)
7.11.2.1.
The dimensionality of a space is the number of numbers needed to specify a point.
7.11.2.2.
A vector in that space has exactly that many ordered numbers in its specification
7.11.2.3.
Examples are position, velocity, acceleration, force, momentum
7.11.2.4.
The components of a vector must transform exactly like the coordinates under a transformation.
7.11.3. Matrix: A two dimensional array of numbers Cij where i is the row and j is the column
7.11.3.1.
A matrix is often used to perform a linear transformation on a vector
7.11.3.2.
Also used to solve a set of simultaneous linear equations. <example of rotations>
7.11.3.3.
Commutation of matrices – a matrix as a linear operator [A,B] = AB – BA
7.11.4. A scalar is a tensor of rank 0, a vector is a tensor of rank 1, a matrix is a tensor of rank 2
7.11.5. Operations with vectors:
7.11.5.1.
Graphical (as used in high school)
7.11.5.2.
i, j, k unit vectors as used in some engineering texts (do not use this notation)
7.11.5.3.
r = (x, y, z) or (x1, x2, x3) or simply as xi or for example (3, -2, 5)
7.11.5.4.
Linear Vector Space (LVS): Addition, subtraction, & multiplication by a scalar <examples>
7.11.5.5.
Metric Space (LVS with a scalar product): Scalar product A B = |A| |B| cos
7.11.5.5.1. Note that this contains the Pythagorean theorem
7.11.5.5.2. Thus A B = Ax Bx + Ay By + Az Bz = g ABwhere generally the metric can be functions of x
7.11.6. More Advanced foundations of vector notation for LVS (Linear Vector Spaces)
7.11.6.1.
Vectors are denoted | a, b, c …> for the space and < a, b, c …| for the dual space.
7.11.6.1.1. A finite or infinite dimensional LVS is spanned by an orthonormal set | i > where i ranges over indices
7.11.6.1.2. The scalar product is then given by < i | j > = ij or perhaps with a metric g as < i |g| j >
7.11.6.1.3. The decomposition of unity is given by 1 = | i > < Ii > where represents a sum or integral over i
7.11.6.2.
Abstract operations such as | x > = L | y > can be put into a given basis by the decomposition of unity as
7.11.6.2.1. < j | x > = < j | L | i > < Ii > | y > which gives the familiar form: xj = i Lji yi
7.11.6.3.
A LVS with a commutator defined is a Lie Algebra: [Li, Lj] = cijk Lk
7.11.6.3.1.1.
(where c is antisymmetric and obeys the Jacobi identity)
7.11.6.3.2. A Lie algebra generates the transformations of a Lie (continuous group) via G(s) = esL (s = real #)
48
8. Some Useful Values
Energy & Power Related Concepts
Energy Units
1 Joule = F*d= Newton * Meter
1 K Calorie = 4186 J
= heat necessary to raise the temp of 1 Kg of H2O by 1 deg C
1 calorie
= heat necessary to raise the temp of 1 gram of H20 by 1 deg C
1 BTU = 1055 J
= heat necessary to raise the temp of 1 lb H20 by 1 deg F
1 KWHR = 3.6 E6 J
= 1 Kilo Watt of power times 1 hour
1 Therm = 1E5 BTU
= heat content of 100 ft3 of natural gas
1 Kilo Ton = 1E12 cal
= energy in one thousand tons of TNT
1 Barrel Oil = 5.6E6 BTU
= energy of crude oil per barrel
1 KG of chemical fuel = 1E7 – 5E7 J
energy range of chemical processes (bread 1100KC/lb to Nat Gas)
1 KG of nuclear fuel = 1E14
= fusion or fission process
1 KG of matter-antimatter = 1E17 = total matter antimatter annihilation
1 kitchen match = 1 BTU
1 ev = 1.6E-19 J
= energy from 1 electron falling between 1 Volt potential difference
Power Units
1 Watt = 1 J / s
1 HP = 745.7 W
= power from 1 horse
1 person’s energy per day
= 2000KC/day = about 100W
1 person’s maximum power
= 100 W continuous, 400 W peak
Solar power per area
1.4 KW / m2
= Max value above atmosphere
1KW / m2
= Max at equator at noon on a clear day
200 W / m2
= US & SC year round Average (day, night, rain sun)
1.7E17 W / whole earth
= total solar power to the earth
Wood gives 2 tons/acre / year
= 0.2 W /m2 thus is 1% efficient
Average US person total energy very approximately is 18 KW / person
Person walking 260 BTU/mile
Person on bike 80 BTU/mile
Automobile
10,000 BTU/mile if 1 occupant only
Efficiency (approximate values)
Agriculture
Primitive use is 0.2 to 0.5 C to get 1 C
Modern use is 15 C to get 1 C
Heat Pump (eg in SC)
200%
Oil or NG furnace
85%
Passive Solar
45%
Active Solar Cells to Elec.
10%
Incandescent Light
3%
Fluorescent light
15%
Notes:
Doubling Time:
Population
US
The Earth
State of SC
% * t = 72
300 Million (October 2007)
6.5 Billion (2007)
4 Million
49
9. How to Best Process This Material as a Physics Course:
This course is not a ‘tech school’ course but a demanding and hopefully enriching major university course developing a broad base of
technical knowledge and insights, coupled with new methods of thinking. Specifically we seek:
1. To learn the foundational laws of nature and science that underlie, not just Physics, but by virtue of being foundational, underlie also
Chemistry, Biology, Geology, Engineering, Biology, Medicine, Health Science, and other scientific fields.
2. To learn specifically the fundamental concepts, their definitions, their experimental and theoretical relationships among one another
(equations) and fundamental values and associated constants and units.
3. To become experienced in estimation, numerical uncertainty, order of magnitude estimation, and problem solving.
4. To learn how ‘science’ operates: the interplay of theory and experiment and the linking of a model, with confirmation of existing data
and prediction of new data.
5. To experience mathematics as a tool of theoretical modeling, prediction, measurement – ie with mathematics as a language.
6. To learn how to think analytically and synthetically: what to question and how, and how to identify what should be generally
accepted and thus questioned less often. To build ability and an associated confidence in reasoning in new domains.
7. To learn a sense of history, and the role of science and technology in the historical evolution of man and civilization.
8. To understand how the human view of nature comprises a limited domain: m, a, v, x, t, g, color/freq, sound etc. Especially how our
senses translate stimulus and register its logarithm.
8. Specifically we seek to understand this underlying theoretical structure along with its successes and current limitations in a holistic
manner.
Recommendation of how to learn the most with the least effort:
1. Preview material prior to each class: We will follow the text and the syllabus and specifically the typed lecture notes available on the
Web – Print this and bring it to class each time. Prior to each class, preview the material for the next class even if just for 10 minutes.
That way, you know what is in the book and my typed notes, and what things are important about those concepts. One will get an
overview of the material to be covered and this makes it far easier to rapidly assimilate the lecture and to take notes that complement
(and do not reproduce) the text.
2. Attend all classes for the entire period: I am not impressed with the taking of voluminous notes, but rather the student who listens,
absorbs, and assimilates the lecture. Your notes should indicate where the concentration areas, important concepts, things to be ignored,
and what will be on the tests. Really listen with full attention.
3. After class but that same day, create a nice set of notes: With your class notes in front of you, your text open to the class material,
with your memory of your pre-class reading of the text, the class notes on the web site, and the knowledge learned in class, then make a
set of clear neat notes that condenses the class lecture and the text. Use the class web site to keep up to date and print out older pertinent
exams etc.
4. Review these condensed notes prior to each exam: Use the condensed notes to review for the exam along with the text. Practice
taking the older tests where pertinent. It is always best to study with other students and share information and to explain concepts to
others. It is a fact that if you explain something and teach someone else, you will learn more in the process than they do, so never
hesitate to help others. In the process of teaching, you will formulate the concepts and relationships more clearly.
5. After each of the four tests, classify your errors into types such as (a) arithmetic or algebraic mistake in calculation, (b) forgot
formula, (c) could not convert the word explanation or setting into a mathematical setting, (d) carelessness (eg marking the wrong
question or alternative.
6. Never miss class if possible – attendance is required. Never cut a test if possible all tests are required.
50