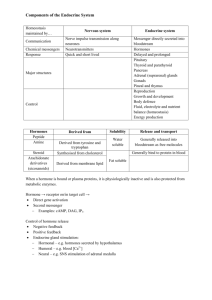
Endocrinology is the study of chemical communication systems that
advertisement

ENDOCRINOLOGY is the study of chemical communication systems that provide the means to control a huge number of physiologic processes. Like other communication networks, endocrine systems contain transmitters, signals and receivers that are called, respectively hormone producing cells, hormones and receptors. The first step in understanding endocrinology is to explore the meaning of such terms as hormone, receptor and target cell, and to obtain an understanding of how chemical communication is controlled. Overview of Endocrinology -----------------------------------------------------------------------How is it that humans and animals maintain quite constant blood concentrations of glucose throughout their lives despite wildly varying frequencies of meals? If your blood glucose concentration drops much below 1 mg per ml, your neurons will begin to misbehave, leading ultimately to coma and death. Yet skipping breakfast is rarely life-threatening. The answer is that a battery of chemical messengers - hormones - are secreted into blood in response to rises and falls in blood glucose concentration and stimulate metabolic pathways that pull glucose concentrations back into the normal range. Two systems control all physiologic processes: * The nervous system exerts point-to-point control through nerves, similar to sending messages by conventional telephone. Nervous control is electrical in nature and fast. * The endocrine system broadcasts its hormonal messages to essentially all cells by secretion into blood and extracellular fluid. Like a radio broadcast, it requires a receiver to get the message - in the case of endocrine messages, cells must bear a receptor for the hormone being broadcast in order to respond. As will be repeatedly demonstrated, the nervous and endocrine systems often act together to regulate physiology. Indeed, some neurons function as endocrine cells. Endocrinology is the study of hormones, their receptors and the intracellular signalling pathways they invoke. Distinct endocrine organs are scattered throughout the body. These are organs that are largely or at least famously devoted to secretion of hormones, and no introduction to endocrinology would be complete without some kind of endocrine organ "map" such as that below: In addition to the classical endocrine organs, many other cells in the body secrete hormones. Myocytes in the atria of the heart and scattered epithelial cells in the stomach and small intestine are examples of what is sometimes called the "diffuse" endocrine system. If the term hormone is defined broadly to include all secreted chemical messengers, then virtually all cells can be considered part of the endocrine system. A final introductory comment is warranted. Pursuit of an understanding of endocrinology is complicated by several of its principles: * All pathophysiologic events are influenced by the endocrine milieu: There are no cell types, organs or processes that are not influenced - often profoundly - by hormone signaling. * All "large" physiologic effects are mediated by multiple hormones acting in concert: Normal growth from birth to adulthood, for example, is surely dependent on growth hormone, but thyroid hormones, insulin-like growth factor-1, glucocorticoids and several other hormones are also critically involved in this process. * There are many hormones known and little doubt that others remain to be discovered. Consequently, endocrinology is presented here in two ways. First, the major endocrine organs and the hormones they produce are introduced, with delineation of major effects. Second, an integrated approach to understanding the multi-hormone control of several important phenomena is provided. Importantly, a considerable amount of endocrinology is incorporated into other sections. Gastrointestinal hormones, for example, are discussed throughout the section on pathophysiology of the digestive system. Hormones, Receptors and Target Cells -----------------------------------------------------------------------What exactly are hormones and how are they different from "non-hormones"? Hormones are chemical messengers secreted into blood or extracellular fluid by one cell that affect the functioning of other cells. Most hormones circulate in blood, coming into contact with essentially all cells. However, a given hormone usually affects only a limited number of cells, which are called target cells. A target cell responds to a hormone because it bears receptors for the hormone. In other words, a particular cell is a target cell for a hormone if it contains functional receptors for that hormone, and cells which do not have such a receptor cannot be influenced directly by that hormone. Reception of a radio broadcast provides a good analogy. Everyone within range of a transmitter for National Public Radio is exposed to that signal (even if they don't contribute!). However, in order to be a NPR target and thus influenced directly by their broadcasts, you have to have a receiver tuned to that frequency. XxxxxxxxxxxxxxxxxxXXXXXXXXXXFOTO 1 Hormone receptors are found either exposed on the surface of the cell or within the cell, depending on the type of hormone. In very basic terms, binding of hormone to receptor triggers a cascade of reactions within the cell that affects function. Additional details about receptor structure and function are provided in the section on hormone mechanism of action. A traditional part of the definition of hormones described them as being secreted into blood and affecting cells at distant sites. However, many of the hormones known to act in that manner have been shown to also affect neighboring cells or even have effects on the same cells that secreted the hormone. Nonetheless, it is useful to be able to describe how the signal is distributed for a particular hormonal pathway, and three actions are defined: * Endocrine action: the hormone is distributed in blood and binds to distant target cells. * Paracrine action: the hormone acts locally by diffusing from its source to target cells in the neighborhood. * Autocrine action: the hormone acts on the same cell that produced it. XXXfoto 2 Two important terms are used to refer to molecules that bind to the hormone-binding sites of receptors: * Agonists are molecules that bind the receptor and induce all the post-receptor events that lead to a biologic effect. In other words, they act like the "normal" hormone, although perhaps more or less potently. Natural hormones are themselves agonists and, in many cases, more than one distinct hormone binds to the same receptor. For a given receptor, different agonists can have dramatically different potencies. * Antagonists are molecules that bind the receptor and block binding of the agonist, but fail to trigger intracellular signalling events. Antagonists are like certain types of bureaucrats - they don't themselves perform useful work, but block the activities of those that do have the capacity to contribute. Hormone antagonists are widely used as drugs. Finally, a comment on the names given hormones and what some have called the tyranny of terminology. Hormones are inevitably named shortly after their discovery, when understanding is necessarily rudimentary. They are often named for the first physiologic effect observed or for their major site of synthesis. As knowledge and understanding of the hormone grow, the original name often appears inappropriate or too restrictive, but it has become entrenched in the literature and is rarely changed. In other situations, a single hormone will be referred to by more than one name. The problem is that the names given to hormones often end up being either confusing or misleading. The solution is to view names as identifiers rather than strict guidelines to source or function. Hormone Chemistry, Synthesis and Elimination -----------------------------------------------------------------------Nature uses a diverse spectrum of molecules as hormones, and knowing the basic structure of a hormone imparts considerable knowledge about its receptor and mechanism of action. Additionally, the simpler structures can often be exploited to generate similar molecules agonists and antagonists - that are therapeutically valuable. Like all molecules, hormones are synthesized, exist in a biologically active state for a time, and then degrade or are destroyed. Again, having an appreciation for the "halflife" and mode of elimination of a hormone aids in understanding its role in physiology and is critical when using hormones as drugs. Most commonly, hormones are categorized into four structural groups, with members of each group having many properties in common: * * * * Peptides and proteins Steroids Amino acid derivatives Fatty acid derivatives - Eicosanoids Peptides and Proteins XXXXXXXXXXXXXXFOTO 4 Peptide and protein hormones are, of course, products of translation. They vary considerably in size and post-translational modifications, ranging from peptides as short as three amino acids to large, multisubunit glycoproteins. Many protein hormones are synthesized as prohormones, then proteolytically clipped to generate their mature form. In other cases, the hormone is originally embedded within the sequence of a larger precursor, then released by multiple proteolytic cleavages. Peptide hormones are synthesized in endoplasmic reticulum, transferred to the Golgi and packaged into secretory vesicles for export. They can be secreted by one of two pathways: * Regulated secretion: The cell stores hormone in secretory granules and releases them in "bursts" when stimulated. This is the most commonly used pathway and allows cells to secrete a large amount of hormone over a short period of time. * Constitutive secretion: The cell does not store hormone, but secretes it from secretory vesicles as it is synthesized. Most peptide hormones circulate unbound to other proteins, but exceptions exist; for example, insulin-like growth factor-1 binds to one of several binding proteins. In general, the halflife of circulating peptide hormones is only a few minutes. Steroids Steroids are lipids and, more specifically, derivatives of cholesterol. Examples include the sex steroids such as testosterone and adrenal steroids such as cortisol. The first and rate-limiting step in the synthesis of all steroid hormones is conversion of cholesterol to pregnenolone, which is illustrated here to demonstate the system of numbering rings and carbons for identification of different steroid hormones. Pregnenolone is formed on the inner membrane of mitochondria then shuttled back and forth between mitochondrion and the endoplasmic reticulum for further enzymatic transformations involved in synthesis of derivative steroid hormones. Newly synthesized steroid hormones are rapidly secreted from the cell, with little if any storage. Increases in secretion reflect accelerated rates of synthesis. Following secretion, all steroids bind to some extent to plasma proteins. This binding is often low affinity and nonspecific (e.g. to albumin), but some steroids are transported by specific binding proteins, which clearly affects their halflife and rate of elimination. Steroid hormones are typically eliminated by inactivating metabolic transformations and excretion in urine or bile. Amino Acid Derivatives There are two groups of hormones derived from the amino acid tyrosine: * Thyroid hormones are basically a "double" tyrosine with the critical incorporation of 3 or 4 iodine atoms. * Catecholamines include epinephrine and norepinephrine, which are used as both hormones and neurotransmitters. The pathways to synthesis of these hormones is provided in the sections on the thyroid gland and the adrenal medulla. The circulating halflife of thyroid hormones is on the order of a few days. They are inactivated primarily by intracellular deiodinases. Catecholamines, on the other hand, are rapidly degraded, with circulating halflives of only a few minutes. Two other amino acids are used for synthesis of hormones: * * Tryptophan is the precursor to serotonin and the pineal hormone melatonin Glutamic acid is converted to histamine Fatty Acid Derivatives - Eicosanoids Eicosanoids are a large group of molecules derived from polyunsaturated fatty acids. The principal groups of hormones of this class are prostaglandins, prostacyclins, leukotrienes and thromboxanes. Arachadonic acid is the most abundant precursor for these hormones. Stores of arachadonic acid are present in membrane lipids and released through the action of various lipases. The specific eicosanoids synthesized by a cell are dictated by the battery of processing enzymes expressed in that cell. These hormones are rapidly inactivated by being metabolized, and are typically active for only a few seconds. Control of Endocrine Activity ------------------------------------------------------------------------ The physiologic effects of hormones depend largely on their concentration in blood and extracellular fluid. Almost inevitably, disease results when hormone concentrations are either too high or too low, and precise control over circulating concentrations of hormones is therefore crucial. The concentration of hormone as seen by target cells is determined by three factors: * Rate of production: Synthesis and secretion of hormones are the most highly regulated aspect of endocrine control. Such control is mediated by positive and negative feedback circuits, as described below in more detail. * Rate of delivery: An example of this effect is blood flow to a target organ or group of target cells - high blood flow delivers more hormone than low blood flow. * Rate of degradation and elimination: Hormones, like all biomolecules, have characteristic rates of decay, and are metabolized and excreted from the body through several routes. Shutting off secretion of a hormone that has a very short halflife causes circulating hormone concentration to plummet, but if a hormone's biological halflife is long, effective concentrations persist for some time after secretion ceases. FOTO 4XXXXXXXXXXXXXXXXXXXXXXXXXXXX Feedback Control of Hormone Production Feedback circuits are at the root of most control mechanisms in physiology, and are particularly prominent in the endocrine system. Instances of positive feedback certainly occur, but negative feedback is much more common. Negative feedback is seen when the output of a pathway inhibits inputs to the pathway. The heating system in your home is a simple negative feedback circuit. When the furnace produces enough heat to elevate temperature above the set point of the thermostat, the thermostat is triggered and shuts off the furnace (heat is feeding back negatively on the source of heat). When temperature drops back below the set point, negative feedback is gone, and the furnace comes back on. Feedback loops are used extensively to regulate secretion of hormones in the hypothalamicpituitary axis. An important example of a negative feedback loop is seen in control of thyroid hormone secretion. The thyroid hormones thyroxine and triiodothyronine ("T4 and T3") are synthesized and secreted by thyroid glands and affect metabolism throughout the body. The basic mechanisms for control in this system (illustrated to the right) are: * Neurons in the hypothalamus secrete thyroid releasing hormone (TRH), which stimulates cells in the anterior pituitary to secrete thyroid-stimulating hormone (TSH). * TSH binds to receptors on epithelial cells in the thyroid gland, stimulating synthesis and secretion of thyroid hormones, which affect probably all cells in the body. * When blood concentrations of thyroid hormones increase above a certain threshold, TRHsecreting neurons in the hypothalamus are inhibited and stop secreting TRH. This is an example of "negative feedback". Inhibition of TRH secretion leads to shut-off of TSH secretion, which leads to shut-off of thyroid hormone secretion. As thyroid hormone levels decay below the threshold, negative feedback is relieved, TRH secretion starts again, leading to TSH secretion ... Another type of feedback is seen in endocrine systems that regulate concentrations of blood components such as glucose. Drink a glass of milk or eat a candy bar and the following (simplified) series of events will occur: * Glucose from the ingested lactose or sucrose is absorbed in the intestine and the level of glucose in blood rises. * Elevation of blood glucose concentration stimulates endocrine cells in the pancreas to release insulin. * Insulin has the major effect of facilitating entry of glucose into many cells of the body as a result, blood glucose levels fall. * When the level of blood glucose falls sufficiently, the stimulus for insulin release disappears and insulin is no longer secreted. Numerous other examples of specific endocrine feedback circuits are presented in the sections on specific hormones or endocrine organs. Hormone Profiles: Concentrations Over Time XXXXXXXXFOTO5 One important consequence of the feedback controls that govern hormone concentrations and the fact that hormones have a limited lifespan or halflife is that most hormones are secreted in "pulses". The following graph depicts concentrations of luteinizing hormone in the blood of a female dog over a period of 8 hours, with samples collected every 15 minutes: The pulsatile nature of luteinizing hormone secretion in this animal is evident. Luteinizing hormone is secreted from the anterior pituitary and critically involved in reproductive function; the frequency and amplitude of pulses are quite different at different stages of the reproductive cycle. With reference to clinical endocrinology, examination of the graph should also demonstrate the caution necessary in interpreting endocrine data based on isolated samples. A pulsatile pattern of secretion is seen for virtually all hormones, with variations in pulse characteristics that reflect specific physiologic states. In addition to the short-term pulses discussed here, longer-term temporal oscillations or endocrine rhythms are also commonly observed and undoubtedly important in both normal and pathologic states. Steroidogenesis -----------------------------------------------------------------------Steroid hormones are derivatives of cholesterol that are synthesized by a variety of tissues, most prominently the adrenal gland and gonads. The cholesterol precursor comes from cholesterol synthesized within the cell from acetate, from cholesterol ester stores in intracellular lipid droplets or from uptake of cholesterol-containing low density lipoproteins. Lipoproteins taken up from plasma are most important when steroidogenic cells are chronically stimulated. The basic cyclopentanoperhydrophenanthrene ring structure and carbon numbering system of all steroid hormones is depicted to the right, using pregnenolone as an example. Pregnenolone is an example of what is called a "C-21 steroid" because it has 21 carbons. Similarly, a steroid such as testosterone (see below) is referred to as a "C-19 steroid". Biosynthesis of steroid hormones requires a battery of oxidative enzymes located in both mitochondria and endoplasmic reticulum. The rate-limiting step in this process is the transport of free cholesterol from the cytoplasm into mitochondria. Within mitochondria, cholesterol is converted to pregnenolone by an enzyme in the inner membrane called CYP11A1. Pregnenolone itself is not a hormone, but is the immediate precursor for the synthesis of all of the steroid hormones. The following table delineates the enzymes required to synthesize the major classes of steroid hormones. Common name "Old" name Current name Side-chain cleavage enzyme; desmolase P450SCC CYP11A1 3 beta-hydroxysteroid dehydrogenase 3 beta-HSD 3 beta-HSD 17 alpha-hydroxylase/17,20 lyase P450C17 CYP17 21-hydroxylase P450C21 CYP21A2 11 beta-hydroxylase P450C11 CYP11B1 Aldosterone synthase P450C11AS CYP11B2 Aromatase P450aro CYP19 Typically, endocrinologists classify steroid hormones into five groups of molecules, based primarily on the receptor to which they bind: * Glucocorticoids; cortisol is the major representative in most mammals * * * * Mineralocorticoids; aldosterone being most prominent Androgens such as testosterone Estrogens, including estrodiol and estrone Progestogens (also known a progestins) such as progesterone The biosynthetic pathways for major representatives of these classes of steroid hormones is depicted in the following diagram. Be aware that a variety of related molecules exist, some of which may have significant effects, particularly in certain pathologic conditions. Mechanisms of Hormone Action: Introduction and Index --------------------------------------------------------Immediately after discovery of a new hormone, a majority of effort is devoted to delineating its sites of synthesis and target cells, and in characterizing the myriad of physiologic responses it invokes. An equally important area of study is to determine precisely how the hormone acts to change the physiologic state of its target cells - its mechanism of action. Understanding mechanism of action is itself a broad task, encompassing structure and function of the receptor, how the bound receptor transduces a signal inside the cell and the end effectors of that signal. This information is not only of great interest to basic science, but critical to understanding and treating diseases of the endocrine system, and in using hormones as drugs. How Do Hormones Change Their Target Cells? -----------------------------------------------------------------------Hormones are chemical messengers that invoke profound changes within target cells. How is this accomplished? There are two fundamental mechanisms by which such changes occur: * Activation of enzymes and other dynamic molecules: Most enzymes shuttle between conformational states that are catalytically active versus inactive, on versus off. Many hormones affect their target cells by inducing such transitions, usually causing an activation of one of more enzymes. Because enzymes are catalytic and often serve to activate additional enzymes, a seemingly small change induced by hormone-receptor binding can lead to widespread consequences within the cell. * Modulation of gene expression: Stimulating transcription of a group of genes clearly can alter a cell's phenotype by leading to a burst of synthesis of new proteins. Similarly, if transcription of a group of previously active genes is shut off, the corresponding proteins will soon disappear from the cell. More specifically, when a receptor becomes bound to a hormone, it undergoes a conformational change which allows it to interact productively with other components of the cells, leading ultimately to an alteration in the physiologic state of the cell. Considerable information about a how a hormone acts can be gained by knowing the type of receptor it uses. Despite the molecular diversity of hormones, all hormone receptors can be categorized into one of two types, based on their location within the cell: Location of Receptor Classes of Hormones Principle Mechanism of Action Cell surface receptors (plasma membrane) Proteins and peptides, catecholamines and eicosanoids Generation of second messengers which alter the activity of other molecules usually enzymes - within the cell Intracellular receptors (cytoplasm and/or nucleus) Steroids and thyroid hormones Alter transcriptional activity of responsive genes Thus, if introduced to a new steroid hormone, one can quickly deduce that it has an intracellular receptor and acts upon its target cells by affecting transcription. XXXXXXXXXXXXXfoto 6 Mechanism of Action: Hormones with Cell Surface Receptors -------------------Protein and peptide hormones, catecholamines like epinephrine, and eicosanoids such as prostaglandins find their receptors decorating the plasma membrane of target cells. Binding of hormone to receptor initiates a series of events which leads to generation of so-called second messengers within the cell (the hormone is the first messenger). The second messengers then trigger a series of molecular interactions that alter the physiologic state of the cell. Another term used to describe this entire process is signal transduction. Structure of Cell Surface Receptors Cell surface receptors are integral membrane proteins and, as such, have regions that contribute to three basic domains: * Extracellular domains: Some of the residues exposed to the outside of the cell interact with and bind the hormone - another term for these regions is the ligand-binding domain. * Transmembrane domains: Hydrophobic stretches of amino acids are "comfortable" in the lipid bilayer and serve to anchor the receptor in the membrane. * Cytoplasmic or intracellular domains: Tails or loops of the receptor that are within the cytoplasm react to hormone binding by interacting in some way with other molecules, leading to generation of second messengers. Cytoplasmic residues of the receptor are thus the effector region of the molecule. XXXXXXXXXXXXXXfoto 7 Several distinctive variations in receptor structure have been identified. As depicted below, some receptors are simple, single-pass proteins; many growth factor receptors take this form. Others, such as the receptor for insulin, have more than one subunit. Another class, which includes the beta-adrenergic receptor, is threaded through the membrane seven times. Receptor molecules are neither isolated by themselves nor fixed in one location of the plasma membrane. In some cases, other integral membrane proteins interact with the receptor to modulate its activity. Some types of receptors cluster together in the membrane after binding hormone. Finally, as elaborated below, interaction of the hormone-bound receptor with other membrane or cytoplasmic proteins is the key to generation of second messengers and transduction of the hormonal signal. Second Messenger Systems Consider what would happen if, late at night, you noticed a building on fire. Hopefully, you would dial 911 or a similar emergency number. You would inform the dispatcher of the fire, and the dispatcher would, in turn, contact and "activate" a number of firemen. The firefighters would then rapidly go to work pouring water on the fire, setting up roadblocks and the like. They would also probably activate other "players", such as police and fire investigators that would come in later to try and determine the cause of the fire. Importantly, once the fire is out (or the building totally destroyed), the firemen go back to the station and to sleep. The community response to a fire is, at least in some ways, analogous to a second messenger system involved in a hormone's action. In the scenario described, you are the "first messenger", the dispatcher is "receptor", the firefighters are "second messengers". Currently, four second messenger systems are recognized in cells, as summarized in the table below. Note that not only do multiple hormones utilize the same second messenger system, but a single hormone can utilize more than one system. Understanding how cells integrate signals from several hormones into a coherent biological response remains a challenge. Xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxfoto 8 Second MesXXXXXXxxsengxxxxer Examples of Hormones Which Utilize This System Cyclic AMP Epinephrine and norepinephrine, glucagon, luteinizing hormone, follicle stimulating hormone, thyroid-stimulating hormone, calcitonin, parathyroid hormone, antidiuretic hormone Protein kinase activityInsulin, growth hormone, prolactin, oxytocin, erythropoietin, several growth factors Calcium and/or phosphoinositides Epinephrine and norepinephrine, angiotensin II, antidiuretic hormone, gonadotropin-releasing hormone, thyroid-releasing hormone. Cyclic GMP Atrial naturetic hormone, nitric oxide In all cases, the seemingly small signal generated by hormone binding its receptor is amplified within the cell into a cascade of actions that changes the cell's physiologic state. Presented below are two examples of second messenger systems commonly used by hormones. The examples used are of glucagon and insulin, both of which ultimately work through a molecular switch involving protein phosphorylation. Be aware that in both cases, a very complex system is being simplified considerably. Cyclic AMP Second Messenger Systems Cyclic adenosine monophosphate (cAMP) is a nucleotide generated from ATP through the action of the enzyme adenylate cyclase. The intracellular concentration of cAMP is increased or decreased by a variety of hormones and such fluctuations affect a variety of cellular processes. One prominent and important effect of elevated concentrations of cAMP is activation of a cAMP-dependent protein kinase called protein kinase A. Protein kinase A is nominally in an catalytically-inactive state, but becomes active when it binds cAMP. Upon activation, protein kinase A phosphorylates a number of other proteins, many of which are themselves enzymes that are either activated or suppressed by being phosphorylated. Such changes in enzymatic activity within the cell clearly alter its state. Now, let's put this information together to understand the mechanism of action of a hormone like glucagon: * Glucagon binds its receptor in the plasma membrane of target cells (e.g. hepatocytes). * Bound receptor interacts with and, through a set of G proteins, turns on adenylate cyclase, which is also an integral membrane protein. * Activated adenylate cyclase begins to convert ATP to cyclic AMP, resulting in an elevated intracellular concentration of cAMP. * High levels of cAMP in the cytosol make it probable that protein kinase A will be bound by cAMP and therefore catalytically active. * Active protein kinase A "runs around the cell" adding phosphates to other enzymes, thereby changing their conformation and modulating their catalytic activity - - - abracadabra, the cell has been changed! * Levels of cAMP decrease due to destruction by cAMP-phosphodiesterase and the inactivation of adenylate cyclase. In the above example, the hormone's action was to modify the activity of pre-existing components in the cell. Elevations in cAMP also have important effects on transcription of certain genes. Tyrosine Kinase Second Messenger Systems The receptors for several protein hormones are themselves protein kinases which are switched on by binding of hormone. The kinase activity associated with such receptors results in phosphorylation of tyrosine residues on other proteins. Insulin is an example of a hormone whose receptor is a tyrosine kinase. The hormone binds to domains exposed on the cell's surface, resulting in a conformational change that activates kinase domains located in the cytoplasmic regions of the receptor. In many cases, the receptor phosphorylates itself as part of the kinase activation process. The activated receptor phosphorylates a variety of intracellular targets, many of which are enzymes that become activated or are inactivated upon phosphorylation. The animation to the right is meant to depict a tyrosine kinase receptor like that used by insulin. Following binding of hormone, the receptor undergoes a conformational change, phosphorylates itself, then phosphorylates a variety of intracellular targets. As was seen with cAMP second messenger systems, activation of receptor tyrosine kinases leads to rapid modulation in a number of target proteins within the cell. Interestingly, some of the targets of receptor kinases are protein phosphatases which, upon activation by receptor tyrosine kinase, become competent to remove phosphates from other proteins and alter their activity. Again, a seemingly small change due to hormone binding is amplified into a multitude of effects within the cell. In some cases, binding of hormone to a surface receptor induces a tyrosine kinase cascade even through the receptor is not itself a tyrosine kinase. The growth hormone receptor is one example of such a system - the interaction of growth hormone with its receptor leads to activation of cytoplasmic tyrosine kinases, with results conceptually similar to that seen with receptor kinases. Fate of the Hormone-Receptor Complex Normal cell function depends upon second messenger cascades being transient events. Indeed, a number of cancers are associated with receptors that continually stimulate second messenger systems. One important part of negative regulation on hormone action is that cell surface receptors are internalized. In many cases, internalization is stimuated by hormone binding. Internalization occurs by endocytosis through structures called coated pits. The resulting endosomes (sometimes called "receptosomes") may fuse with lysosomes, leading to destruction of the receptor and hormone. In other cases, it appears that the hormone dissociates and the receptor is recycled by fusion of the endosome back into the plasma membrane. Mechanism of Action: Hormones with Intracellular Receptors -----------------------------------------------------------------------Receptors for steroid and thyroid hormones are located inside target cells, in the cytoplasm or nucleus, and function as ligand-dependent transcription factors. That is to say, the hormone-receptor complex binds to promoter regions of responsive genes and stimulate or sometimes inhibit transcription from those genes. Thus, the mechanism of action of these hormones is to modulate gene expression in target cells. By selectively affecting transcription from a battery of genes, the concentration of those respective proteins are altered, which clearly can change the phenotype of the cell. Structure of Intracellular Receptors Steroid and thyroid hormone receptors are members of a large group ("superfamily") of transcription factors. In some cases, multiple forms of a given receptor are expressed in cells, adding to the complexity of the response. All of these receptors are composed of a single polypeptide chain that has, in the simplist analysis, three distinct domains: * The amino-terminus: In most cases, this region is involved in activating or stimulating transcription by interacting with other components of the transcriptional machinery. The sequence is highly variable among different receptors. * DNA binding domain: Amino acids in this region are responsible for binding of the receptor to specific sequences of DNA. * The carboxy-terminus or ligand-binding domain: This is the region that binds hormone. In addition to these three core domains, two other important regions of the receptor protein are a nuclear localization sequence, which targets the the protein to nucleus, and a dimerization domain, which is responsible for latching two receptors together in a form capable of binding DNA. Hormone-Receptor Binding and Interactions with DNA Being lipids, steroid hormones enter the cell by simple diffusion across the plasma membrane. Thyroid hormones enter the cell by facilitated diffusion. The receptors exist either in the cytoplasm or nucleus, which is where they meet the hormone. When hormone binds to receptor, a characteristic series of events occurs: * Receptor activation is the term used to describe conformational changes in the receptor induced by binding hormone. The major consequence of activation is that the receptor becomes competent to bind DNA. * Activated receptors bind to hormone response elements, which are short specific sequences of DNA which are located in promoters of hormone-responsive genes. In most cases, hormone-receptor complexes bind DNA in pairs, as shown in the figure below. * Transcription from those genes to which the receptor is bound is affected. Most commonly, receptor binding stimulates transcription. The hormone-receptor complex thus functions as a transcription factor. As might be expected, there are a number of variations on the themes described above, depending on the specific receptor in question. For example, in the absense of hormone, some intracellular receptors do bind their hormone response elements loosely and silence transcription, but, when complexed to hormone, become activated and strongly stimulate transcription. Some receptors bind DNA not with another of their kind, but with different intracellular receptor. As a specific example, consider glucocorticoids, a type of steroid hormone that probably affects the physiology of all cells in the body. The image to the right depicts a pair of glucocorticoid receptors (blue and green on the top) bound to their DNA hormone response element (bottom). The two steroid hormones are not visible in this depiction. The consensus sequence of the hormone response element in this case (called a glucocorticoid response element) is GGTACANNNTGTTCT, where N is any nucleotide. The Hypothalamus and Pituitary Gland: Introduction and Index -----------------------------------------It would be difficult to overstate the influence of hypothalamic and pituitary hormones over physiologic processes. The target cells for most of the hormones produced in these tissues are themselves endocrine cells, and a seemingly small initial signal is thus amplified to cause widespread effects on many cells and tissues. The close anatomical and functional relationships between hypothalamus and pituitary force an integrated discussion of these organs. The focus here is to introduce the major hormones produced by these organs, with significant emphasis on how hormone secretion and action are controlled. Links are provided to other sections of text containing additional information on the effects of these hormones. Functional Anatomy of the Hypothalamus and Pituitary Gland ------------------------The hypothalamus is a region of the brain that controls an immense number of bodily functions. It is located in the middle of the base of the brain, and encapsulates the ventral portion of the third ventricle. XxxxxxxxxxxFOTO 9 The pituitary gland, also known as the hypophysis, is a roundish organ that lies immediately beneath the hypothalamus, resting in a depression of the base of the skull called the sella turcica ("Turkish saddle"). In an adult human or sheep, the pituitary is roughly the size and shape of a garbonzo bean. The image to the right, from the Visible Human Project, shows these anatomical relationships in the Visible Woman (click on the image to see a larger, unlabeled image). Careful examination of the pituitary gland reveals that it composed of two distinctive parts: * The anterior pituitary (adenohypophysis) is a classical gland composed predominantly of cells that secrete protein hormones. * The posterior pituitary (neurohypophysis) is not really an organ, but an extension of the hypothalamus. It is composed largely of the axons of hypothalamic neurons which extend downward as a large bundle behind the anterior pituitary. It also forms the so-called pituitary stalk, which appears to suspend the anterior gland from the hypothalamus. XXXXXXXXXXXFOTO 10 The image to the right shows a frontal view of a sheep pituitary gland and hypothalamus. The posterior gland can be seen peeking out behind the anterior gland; pass your mouse cursor over the image for labels (image courtesy of T. Nett). The anterior and posterior pituitary have separate embryological origins. In many mammals, there is also an intermediate lobe (pars intermedia) between the anterior and posterior pituitary. A key to understanding the endocrine relationship between hypothalamus and anterior pituitary is to appreciate the vascular connections between these organs. As will be emphasized in later sections, secretion of hormones from the anterior pituitary is under strict control by hypothalamic hormones. These hypothalamic hormones reach the anterior pituitary through the following route: * A branch of the hypophyseal artery ramifies into a capillary bed in the lower hypothalamus, and hypothalmic hormones destined for the anterior pituitary are secreted into that capillary blood. * Blood from those capillaries drains into hypothalamic-hypophyseal portal veins. Portal veins are defined as veins between two capillary beds; the hypothalamic-hypophyseal portal veins branch again into another series of capillaries within the anterior pituitary. * Capillaries within the anterior pituitary, which carry hormones secreted by that gland, coalesce into veins that drain into the systemic venous blood. Those veins also collect capillary blood from the posterior pituitary gland. This pattern of vascular connections is presented diagramatically below. Note also the hypothalamic-hypophyseal portal vessels in the image of a real pituitary gland seen above. XXXXXXXXXXFOTO 11 The utility of this unconventional vascular system is that minute quantities of hypothalamic hormones are carried in a concentrated form directly to their target cells in the anterior pituitary, and are not diluted out in the systemic circulation. Overview of Hypothalamic and Pituitary Hormones ---------------------The pituitary gland is often portrayed as the "master gland" of the body. Such praise is justified in the sense that the anterior and posterior pituitary secrete a battery of hormones that collectively influence all cells and affect virtually all physiologic processes. XXXXXXXXFOTO 12 The pituitary gland may be king, but the power behind the throne is clearly the hypothalamus. As alluded to in the last section, some of the neurons within the hypothalamus neurosecretory neurons - secrete hormones that strictly control secretion of hormones from the anterior pituitary. The hypothalamic hormones are referred to as releasing hormones and inhibiting hormones, reflecting their influence on anterior pituitary hormones. Hypothalamic releasing and inhibiting hormones are carried directly to the anterior pituitary gland via hypothalamic-hypophyseal portal veins. Specific hypothalamic hormones bind to receptors on specific anterior pituitary cells, modulating the release of the hormone they produce. As an example, thyroid-releasing hormone from the hypothalamus binds to receptors on anterior pituitary cells called thyrotrophs, stimulating them to secrete thyroid-stimulating hormone or TSH. The anterior pituitary hormones enter the systemic circulation and bind to their receptors on other target organs. In the case of TSH, the target organ is the thyroid gland. Clearly, robust control systems must be in place to prevent over or under-secretion of hypothalamic and anterior pituitary hormones. A prominent mechanism for control of the releasing and inhibiting hormones is negative feedback, as described in general in a previous section. Details on the control of specific hypothalamic and anterior pituitary hormones is presented in the discussions of those hormones. The following table summarizes the major hormones synthesized and secreted by the pituitary gland, along with summary statements about their major target organs and physiologic effects. Keep in mind that summaries are just that, and ongoing research continues to delineate additional, sometimes very important effects. Hormone Major target organ(s) Major Physiologic Effects Anterior Pituitary Growth hormone Liver, adipose tissue Promotes growth (indirectly), control of protein, lipid and carbohydrate metabolism Thyroid-stimulating hormone Thyroid gland Stimulates secretion of thyroid hormones Adrenocorticotropic hormone Adrenal gland (cortex) Stimulates secretion of glucocorticoids Prolactin Mammary gland Milk production Luteinizing hormone Ovary and testis Control of reproductive function Follicle-stimulating hormone Ovary and testis Control of reproductive function Posterior Pituitary Antidiuretic hormone Kidney Conservation of body water Oxytocin Ovary and testis Stimulates milk ejection and uterine contractions XXXXXXXXXXXXXXXFOTO 13 Growth Hormone ------------------------Growth hormone, also known as somatotropin, is a protein hormone of about 190 amino acids that is synthesized and secreted by cells called somatotrophs in the anterior pituitary. It is a major participant in control of several complex physiologic processes, including growth and metabolism. Growth hormone is also of considerable interest as a drug used in both humans and animals. Physiologic Effects of Growth Hormone XXXXXXXXFOTO 14 A critical concept in understanding growth hormone activity is that it has two distinct types of effects: * Direct effects are the result of growth hormone binding its receptor on target cells. Fat cells (adipocytes), for example, have growth hormone receptors, and growth hormone stimulates them to break down triglyceride and supresses their ability to take up and accumulate circulating lipids. * Indirect effects are mediated primarily by a insulin-like growth factor-1 (IGF-1), a hormone that is secreted from the liver and other tissues in response to growth hormone. A majority of the growth promoting effects of growth hormone is actually due to IGF-1 acting on its target cells. Keeping this distinction in mind, we can discuss two major roles of growth hormone and its minion IGF-1 in physiology. Effects on Growth Growth is a very complex process, and requires the coordinated action of several hormones. The major role of growth hormone in stimulating body growth is to stimulate the liver and other tissues to secrete IGF-1. IGF-1 stimulates proliferation of chondrocytes (cartilage cells), resulting in bone growth. Growth hormone does seem to have a direct effect on bone growth in stimulating differentiation of chondrocytes. IGF-1 also appears to be the key player in muscle growth. It stimulates both the differentiation and proliferation of myoblasts. It also stimulates amino acid uptake and protein synthesis in muscle and other tissues. Metabolic Effects Growth hormone has important effects on protein, lipid and carbohydrate metabolism. In some cases, a direct effect of growth hormone has been clearly demonstrated, in others, IGF-1 is thought to be the critical mediator, and some cases it appears that both direct and indirect effects are at play. * Protein metabolism: In general, growth hormone stimulates protein anabolism in many tissues. This effect reflects increased amino acid uptake, increased protein synthesis and decreased oxidation of proteins. * Fat metabolism: Growth hormone enhances the utilization of fat by stimulating triglyceride breakdown and oxidation in adipocytes. * Carbohydrate metabolism: Growth hormone is one of a battery of hormones that serves to maintain blood glucose within a normal range. Growth hormone is often said to have anti-insulin activity, because it supresses the abilities of insulin to stimulate uptake of glucose in peripheral tissues and enhance glucose synthesis in the liver. Somewhat paradoxically, administration of growth hormone stimulates insulin secretion, leading to hyperinsulinemia. Control of Growth Hormone Secretion Production of growth hormone is modulated by many factors, including stress, exercise, nutrition, sleep and growth hormone itself. However, its primary controllers are two hypothalamic hormones: XXXXXXXXXXXXXXFOTO 15 * Growth hormone-releasing hormone (GHRH) is a peptide that stimulates both the synthesis and secretion of growth hormone. * Somatostatin (SS) is a peptide produced by several tissues in the body, including the hypothalamus. Somatostatin inhibits growth hormone release in response to GHRH and to other stimulatory factors such as low blood glucose concentration. Growth hormone secretion is also part of a negative feedback loop involving IGF-1. High blood levels of IGF-1 lead to decreased secretion of growth hormone not only by directly suppressing the somatotroph, but by stimulating release of somatostatin from the hypothalamus. Growth hormone also feeds back to inhibit GHRH secretion and probably has a direct (autocrine) inhibitory effect on secretion from the somatotroph. Integration of all the factors that affect growth hormone synthesis and secretion lead to a pulsatile pattern of release. Basal concentrations of growth hormone in blood are very low. In children and young adults, the most intense period of growth hormone release is shortly after the onset of deep sleep. Disease States States of both growth hormone deficiency and excess provide very visible testaments to the role of this hormone in normal physiology. Such disorders can reflect lesions in either the hypothalamus, the pituitary or in target cells. A deficiency state can result not only from a deficiency in production of the hormone, but in the target cell's response to the hormone. Clinically, deficiency in growth hormone or receptor defects are as growth retardation or dwarfism. The manifestation of growth hormone deficiency depends upon the age of onset of the disorder and can result from either heritable or acquired disease. The effect of excessive secretion of growth hormone is also very dependent on the age of onset and is seen as two distinctive disorders: * Giantism is the result of excessive growth hormone secretion that begins in young children or adolescents. It is a very rare disorder, usually resulting from a tumor of somatotropes. One of the most famous giants was a man named Robert Wardlow. He weighed 9 pounds at birth. His parents soon recognized that something was afoot, as his weight at 1 year was 62 pounds. Robert reached an adult weight of 475 pounds and 8 feet 11 inches in height. He was a circus performer prior to his early death. * Acromegaly results from excessive secretion of growth hormone in adults. The onset of this disorder is typically insideous. Clinically, an overgrowth of bone and connective leads to a change in appearance that might be described as having "coarse features". The excessive growth hormone and IGF-1 also lead to metabolic derangements, including glucose intolerance. Pharmaceutical and Biotechnological Uses of Growth Hormone In years past, growth hormone purified from human cadaver pituitaries was used to treat children with severe growth retardation. More recently, the virtually unlimited supply of recombinant growth hormone has lead to several other applications to human and animal populations. Human growth hormone is commonly used to treat children of pathologically short stature. There is concern that this practice will be extended to treatment of essentially normal children so called "enhancement therapy" or growth hormone on demand. Similarly, growth hormone has been used by some to enhance atheletic performance. Although growth hormone therapy is generally safe, it is not as safe as no therapy and does entail unpredictable health risks. Parents that request growth hormone therapy for children of essentially-normal stature are clearly misguided. The role of growth hormone in normal aging remains poorly understood, but some of the cosmetic symptoms of aging appear to be amenable to growth hormone therapy. This is an active area of research, and additional information and recommendations about risks and benefits will undoubtedly surface in the near future. Growth hormone is currently approved and marketed for enhancing milk production in dairy cattle. There is no doubt that administration of bovine somatotropin to lactating cows results in increased milk yield, and, depending on the way the cows are managed, can be an economicallyviable therapy. However, this treatment engenders abundant controversy, even among dairy farmers. One thing that appears clear is that drinking milk from cattle treated with bovine growth hormone does not pose a risk to human health. Another application of growth hormone in animal agriculture is treatment of growing pigs with porcine growth hormone. Such treatment has been demonstrated to significantly stimulate muscle growth and reduce deposition of fat. Thyroid-Stimulating Hormone (Thyrotropin)---Thyroid-stimulating hormone, also known as thyrotropin, is secreted from cells in the anterior pituitary called thyrotrophs, finds its receptors on epithelial cells in the thyroid gland, and stimulates that gland to synthesize and release thyroid hormones. TSH is a glycoprotein hormone composed of two subunits which are non-covalently bound to one another. The alpha subunit of TSH is also present in two other pituitary glycoprotein hormones, follicle-stimulating hormone and luteinizing hormone, and, in primates, in the placental hormone chorionic gonadotropin. Each of these hormones also has a unique beta subunit, which provides receptor specificity. In other words, TSH is composed of alpha subunit bound to the TSH beta subunit, and TSH associates only with its own receptor. Free alpha and beta subunits have essentially no biological activity. XXXXXXXXXXXFOTO 16 The most important controller of TSH secretion is thyroid-releasing hormone. Thyroidreleasing hormone is secreted by hypothalamic neurons into hypothalamic-hypophyseal portal blood, finds its receptors on thyrotrophs in the anterior pituitary and stimulates secretion of TSH. One interesting aspect of thyroid-releasing hormone is that it is only three amino acids long. Its basic sequence is glutamic acid-histidine-proline, although both ends of the peptide are modified. Secretion of thyroid-releasing hormone, and hence, TSH, is inhibited by high blood levels of thyroid hormones in a classical negative feedback loop. Adrenocorticotropic Hormone (ACTH) ------------------------------------------------------------------------ Adrenocorticotropic hormone, as its name implies, stimulates the adrenal cortex. More specifically, it stimulates secretion of glucocorticoids such as cortisol, and has little control over secretion of aldosterone, the other major steroid hormone from the adrenal cortex. Another name for ACTH is corticotropin. ACTH is secreted from the anterior pituitary in response to corticotropin-releasing hormone from the hypothalamus. corticotropin-releasing hormone is secreted in response to many types of stress, which makes sense in view of the "stress management" functions of glucocorticoids. Corticotropin-releasing hormone itself is inhibited by glucocorticoids, making it part of a classical negative feedback loop. XXXXXXXXXXXXXXXX FOTO 17 18 Additional information on the role of ACTH in regulation of adrenal steroid secretion is presented in the sections on the adrenal gland and glucocorticoids. Within the pituitary gland, ACTH is produced in a process that also generates several other hormones. A large precursor protein named proopiomelanocortin (POMC, "Big Mama") is synthesized and proteolytically chopped into several fragments as depicted below. Not all of the cleavages occur in all species and some occur only in the intermediate lobe of the pituitary. The major attributes of the hormones other than ACTH that are produced in this process are summarized as follows: * Lipotropin: Originally described as having weak lipolytic effects, its major importance is as the precursor to beta-endorphin. * Beta-endorphin and Met-enkephalin: Opioid peptides with pain-alleviation and euphoric effects. Melanocyte-stimulating hormone (MSH): Known to control melanin pigmentation in the skin of most vertebrates. Prolactin -----------------------------------------------------------------------Prolactin is a single-chain protein hormone closely related to growth hormone. It is secreted by so-called lactotrophs in the anterior pituitary. It is also synthesized and secreted by a broad range of other cells in the body, most prominently various immune cells, the brain and the decidua of the pregnant uterus. Prolactin is synthesized as a prohormone. Following cleavage of the signal peptide, the length of the mature hormone is between 194 and 199 amino acids, depending on species. Hormore structure is stabilized by three intramolecular disulfide bonds. Physiologic Effects of Prolactin The conventional view of prolactin is that its major target organ is the mammary gland, and stimulating mammary gland development and milk production pretty well define its functions. Such a picture is true as far as goes, but it fails to convey an accurate depiction of this multifunctional hormone. It is difficult to point to a tissue that does not express prolactin receptors, and although the anterior pituitary is the major source of prolactin, the hormone is synthesized and secreted in many other tissues. Overall, several hundred different actions have been reported for prolactin in various species. Some of its major effects are summarized here. Mammary Gland Development, Milk Production and Reproduction XXXXXXXXXXXXFOTO 19 In the 1920's it was found that extracts of the pituitary gland, when injected into virgin rabbits, induced milk production. Subsequent research demonstrated that prolactin has two major roles in milk production: * Prolactin induces lobuloalveolar growth of the mammary gland. Alveoli are the clusters of cells in the mammary gland that actually secrete milk. * Prolactin stimulates lactogenesis or milk production after giving birth. Prolactin, along with cortisol and insulin, act together to stimulate transcription of the genes that encode milk proteins. The critical role of prolactin in lactation has been confirmed in mice with targeted deletions in the prolactin gene. Female mice that are heterozygous for the deleted prolactin gene (and produce roughly half the normal amount of prolactin) show failure to lactate after their first pregnancy. Prolactin also appears important in several non-lactational aspects of reproduction. In some species (rodents, dogs, skunks), prolactin is necessary for maintainance of corpora lutea (ovarian structures that secrete progesterone, the "hormone of pregnancy"). Mice that are homozygous for an inactivated prolactin gene and thus incapable of secreting prolactin are infertile due to defects in ovulation, fertilization, preimplantation development and implantation. Finally, prolactin appears to have stimulatory effects in some species on reproductive or maternal behaviors such as nest building and retrieval of scattered young. Effects on Immune Function The prolactin receptor is widely expressed by immune cells, and some types of lymphocytes synthesize and secrete prolactin. These observations suggest that prolactin may act as an autocrine or paracrine modulator of immune activity. Interestingly, mice with homozygous deletions of the prolactin gene fail to show significant abnormalities in immune responses. A considerable amount of research is in progress to delineate the role of prolactin in normal and pathologic immune responses. It appears that prolactin has a modulatory role in several aspects of immune function, but is not strictly required for these responses. Control of Prolactin Secretion XXXXXXXXXFOTO 20 In contrast to what is seen with all the other pituitary hormones, the hypothalamus tonically suppresses prolactin secretion from the pituitary. In other words, there is usually a hypothalamic "brake" set on the lactotroph, and prolactin is secreted only when the brake is released. If the pituitary stalk is cut, prolactin secretion increases, while secretion of all the other pituitary hormones fall dramatically due to loss of hypothalamic releasing hormones. Dopamine serves as the major prolactin-inhibiting factor or brake on prolactin secretion. Dopamine is secreted into portal blood by hypothalamic neurons, binds to receptors on lactotrophs, and inhibits both the synthesis and secretion of prolactin. Agents and drugs that interfere with dopamine secretion or receptor binding lead to enhanced secretion of prolactin. In addition to tonic inhibition by dopamine, prolactin secretion is positively regulated by several hormones, including thyroid-releasing hormone, gonadotropin-releasing hormone and vasoactive intestinal polypeptide. Stimulation of the nipples and mammary gland, as occurs during nursing, leads to prolactin release. This effect appears to be due to a spinal reflex arc that causes release of prolactin-stimulating hormones from the hypothalamus. Estrogens provide a well-studied positive control over prolactin synthesis and secretion. The increasing blood concentrations of estrogen during late pregnancy appear responsible for the elevated levels of prolactin that are necessary to prepare the mammary gland for lactation at the end of gestation. Disease States Excessive secretion of prolactin - hyperprolactinemia - is a relative common disorder in humans. This condition has numerous causes, including prolactin-secreting tumors and therapy with certain drugs. Common manifestations of hyperprolactinemia in women include amenorrhea (lack of menstrural cycles) and galactorrhea (excessive or spontaneous secretion of milk). Men with hyperprolactinemia typically show hypogonadism, with decreased sex drive, decreased sperm production and impotence. Such men also often show breast enlargement (gynecomastia), but very rarely produce milk. Gonadotropins: Luteinizing and Follicle Stimulating Hormones -----------------------------------------------------------------------Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are called gonadotropins because stimulate the gonads - in males, the testes, and in females, the ovaries. They are not necessary for life, but are essential for reproduction. These two hormones are secreted from cells in the anterior pituitary called gonadotrophs. Most gonadotrophs secrete only LH or FSH, but some appear to secrete both hormones. As describef for thyroid-simulating hormone, LH and FSH are large glycoproteins composed of alpha and beta subunits. The alpha subunit is identical in all three of these anterior pituitary hormones, while the beta subunit is unique and endows each hormone with the ability to bind its own receptor. Physiologic Effects of Gonadotropins Physiologic effects of the gonadotrophins are known only in the ovaries and testes. Together, then regulate many aspects of gonadal function in both males and females. Luteinizing Hormone XXXXXXXXXXXXXXXXXXXFOTO 21 In both sexes, LH stimulates secretion of sex steroids from the gonads. In the testes, LH binds to receptors on Leydig cells, stimulating synthesis and secretion of testosterone. Theca cells in the ovary respond to LH stimulation by secretion of estrogens. In females, ovulation of mature follicles on the ovary is induced by a large burst of LH secretion known as the preovulatory LH surge. Residual cells within ovulated follicles proliferate to form corpora lutea, which secrete the steroid hormones progesterone and estradiol. Progesterone is necessary for maintenance of pregnancy, and, in most mammals, LH is required for continued development and function of corpora lutea. The name luteinizing hormone derives from this effect of inducing luteinization of ovarian follicles. Follicle-Stimulating Hormone As its name implies, FSH stimulates the maturation of ovarian follicles. Administration of FSH to humans and animals induces "superovulation", or development of more than the usual number of mature follicles and hence, an increased number of mature gametes. FSH is also critical for sperm production. It supports the function of Sertoli cells, which in turn support many aspects of sperm cell maturation. Control of Gonadotropin Secretion XXXXXXXXXFOTO 22 The principle regulator of LH and FSH secretion is gonadotropin-releasing hormone or GnRH (also known as LH-releasing hormone). GnRH is a ten amino acid peptide that is synthesized and secreted from hypothalamic neurons and binds to receptors on gonadotrophs. As depicted in the figure to the right, GnRH stimultes secretion of LH, which in turn stimulates gonadal secretion of the sex steroids testosterone, estrogen and progesterone. In a classical negative feedback loop, sex steroids inhibit secretion of GnRH and also appear to have direct negative effects on gonadotrophs. This regulatory loop leads to pulsatile secretion of LH and, to a much lesser extent, FSH. The number of pulses of GnRH and LH varies from a few per day to one or more per hour. In females, pulse frequency is clearly related to stage of the cycle. Numerous hormones influence GnRH secretion, and positive and negative control over GnRH and gonadotropin secretion is actually considerably more complex than depicted in the figure. For example, the gonads secrete at least two additional hormones - inhibin and activin - which selectively inhibit and activate FSH secretion from the pituitary. Disease States Diminished secretion of LH or FSH can result in failure of gonadal function (hypogonadism). This condition is typically manifest in males as failure in production of normal numbers of sperm. In females, cessation of reproductive cycles is commonly observed. Elevated blood levels of gonadotropins usually reflect lack of steroid negative feedback. Removal of the gonads from either males or females, as is commonly done to animals, leads to persistent elevation in LH and FSH. In humans, excessive secretion of FSH and/or LH most commonly the result of gonadal failure or pituitary tumors. In general, elevated levels of gonadotropins per se have no biological effect. Pharmacologic Manipulation of Gonadotropin Secretion Normal patterns of gonadotropin secretion are absolutely required for reproduction, interfering particularly with LH secretion is a widely-used strategy for contraception. contraceptive pills contain a progestin (progesterone-mimicking compound), usually combined an estrogen. As discussed above, progesterone and estrogen inhibit LH secretion, and contraceptives are effective because they inhibit the LH surge that induces ovulation. and Oral with oral Another route to suppressing gonadotropin secretion is to block the GnRH receptor. GnRH receptor antagonists have potent contraceptive effects in both males and females, but have not been widely deployed for that purpose. Antidiuretic Hormone (Vasopressin) -----------------------------------------------------------------------XXXXXXFOTO 23 Roughly 60% of the mass of the body is water, and despite wide variation in the amount of water taken in each day, body water content remains incredibly stable. Such precise control of body water and solute concentrations is a function of several hormones acting on both the kidneys and vascular system, but there is no doubt that antidiuretic hormone is a key player in this process. Antidiuretic hormone, also known as vasopressin, is a nine amino acid peptide secreted from the posterior pituitary. Within hypothalamic neurons, the hormone is packaged in secretory vesicles with a carrier protein called neurophysin, and both are released upon hormone secretion. XXXXXXXXXXFOTO 24 Physiologic Effects of Antidiuretic Hormone Effects on the Kidney The single most important effect of antidiuretic hormone is to conserve body water by reducing the output of urine. A diuretic is an agent that increases the rate of urine formation. Injection of small amounts of antidiuretic hormone into a person or animal results in antidiuresis or decreased formation of urine, and the hormone was named for this effect. Antidiuretic hormone binds to receptors in the distal or collecting tubules of the kidney and promotes reabsorbtion of water back into the circulation. In the absense of antidiuretic hormone, the kidney tubules are virtually impermiable to water, and it flows out as urine. Antidiuretic hormone stimulates water reabsorbtion by stimulating insertion of "water channels" or aquaporins into the membranes of kidney tubules. These channels transport solute-free water through tubular cells and back into blood, leading to a decrease in plasma osmolarity and an increase osmolarity of urine. Effects on the Vascular System In many species, high concentrations of antidiuretic hormone cause widespread constriction of arterioles, which leads to increased arterial pressure. It was for this effect that the name vasopressin was coined. In healthy humans, antidiuretic hormone has minimal pressor effects. Control of Antidiuretic Hormone Secretion The most important variable regulating antidiuretic hormone secretion is plasma osmolarity, or the concentration of solutes in blood. Osmolarity is sensed in the hypothalamus by neurons known as an osmoreceptors, and those neurons, in turn, simulate secretion from the neurons that produce antidiuretic hormone. When plasma osmolarity is below a certain threshold, the osmoreceptors are not activated and antidiuretic hormone secretion is suppressed. When osmolarity increases above the threshold, the ever-alert osmoreceptors recognize this a the cue to stimulate the neurons that secrete antidiuretic hormone. As seen the the figure below, antidiuretic hormone concentrations rise steeply and linearly with increasing plasma osmolarity. Osmotic control of antidiuretic hormone secretion makes perfect sense. Imagine walking across a desert: the sun is beating down and you begin to lose a considerable amount of body water through sweating. Loss of water results in concentration of blood solutes - plasma osmolarity increases. Should you increase urine production in such a situation? Clearly not. Rather, antidiuretic hormone is secreted, allowing almost all the water that would be lost in urine to be reabsorbed and conserved. XXXXXXXXXXFOTO 25 There is an interesting parallel between antidiuretic hormone secretion and thirst. Both phenomena appear to be stimulated by hypothalamic osmoreceptors, although probably not the same ones. The osmotic threshold for antidiuretic hormone secretion is considerably lower than for thirst, as if the hypothalamus is saying "Let's not bother him by invoking thirst unless the situation is bad enough that antidiuretic hormone cannot handle it alone." Secretion of antidiuretic hormone is also simulated by decreases in blood pressure and volume, conditions sensed by stretch receptors in the heart and large arteries. Changes in blood pressure and volume are not nearly as sensitive a stimulator as increased osmolarity, but are nonetheless potent in severe conditions. For example, Loss of 15 or 20% of blood volume by hemorrhage results in massive secretion of antidiuretic hormone. Another potent stimulus of antidiuretic hormone is nausea and vomiting, both of which are controlled by regions in the brain with links to the hypothalamus. Disease States The most common disease of man and animals related to antidiuretic hormone is diabetes insipidus. This condition can arise from either of two situations: * Hypothalamic ("central") diabetes insipidus results from a deficiency in secretion of antidiuretic hormone from the posterior pituitary. Causes of this disease include head trauma, and infections or tumors involving the hypothalamus. * Nephrogenic diabetes insipidus occurs when the kidney is unable to respond to antidiuretic hormone. Most commonly, this results from some type of renal disease, but mutations in the ADH receptor gene or in the gene encoding aquaporin-2 have also been demonstrated in affected humans. The major sign of either type of diabetes insipidus is excessive urine production. Some human patients produce as much as 16 liters of urine per day! If adequate water is available for consumption, the disease is rarely life-threatening, but withholding water can be very dangerous. Hypothalamic diabetes insipidus can be treated with exogenous antidiuretic hormone. Oxytocin -----------------------------------------------------------------------XXXXXXXXXXFOTO 26 27 Oxytocin in a nine amino acid peptide that is synthesized in hypothalamic neurons and transported down axons of the posterior pituitary for secretion into blood. Oxytocin is also secreted within the brain and from a few other tissues, including the ovaries and testes. Oxytocin differs from antidiuretic hormone in two of the nine amino acids. Both hormones are packaged into granules and secreted along with carrier proteins called neurophysins. Physiologic Effects of Oxytocin In years past, oxytocin had the reputation of being an "uncomplicated" hormone, with only a few well-defined activities related to birth and lactation. As has been the case with so many hormones, further research has demonstrated many subtle but profound influences of this little peptide. Nevertheless, it has been best studied in females where it clearly mediates three major effects: * Stimulation of milk ejection (milk letdown): Milk is initially secreted into small sacs within the mammary gland called alveoli, from which it must be ejected for consumption or harvesting. Mammary alveoli are surrounded by smooth muscle (myoepithelial) cells which are a prominant target cell for oxytocin. Oxytocin stimulates contraction of myoepithelial cells, causing milk to be ejected into the ducts and cisterns. * Stimulation of uterine smooth muscle contraction at birth: At the end of gestation, the uterus must contract vigorously and for a prolonged period of time in order to deliver the fetus. During the later stages of gestation, there is an increase in abundance of oxytocin receptors on uterine smooth muscle cells, which is associated with increased "irritability" of the uterus (and sometimes the mother as well). Oxytocin is released during labor when the fetus stimulates the cervix and vagina, and it enhances contraction of uterine smooth muscle to facilitate parturition or birth. In cases where uterine contractions are not sufficient to complete delivery, physicians and veterinarians sometimes administer oxytocin ("pitocin") to further stimulate uterine contractions - great care must be exercised in such situations to assure that the fetus can indeed be delivered and to avoid rupture of the uterus. * Establishment of maternal behavior: Successful reproduction in mammals demands that mothers become attached to and nourish their offspring immediately after birth. It is also important that non-lactating females do not manifest such nurturing behavior. The same events that affect the uterus and mammary gland at the time of birth also affect the brain. During parturition, there is an increase in concentration of oxytocin in cerebrospinal fluid, and oxytocin acting within the brain plays a major role in establishing maternal behavior. Evidence for this role of oxytocin come from two types of experiments. First, infusion of oxytocin into the ventricles of the brain of virgin rats or non-pregnant sheep rapidly induces maternal behavior. Second, administration into the brain of antibodies that neutralize oxytocin or of oxytocin antagonists will prevent mother rats from accepting their pups. Other studies support the contention that this behavioral effect of oxytocin is broadly applicable among mammals. While there is no doubt that oxytocin stimulates all of the effects described above, doubt has recently been cast on its necessity in parturition and maternal behavior. Mice that are unable to secrete oxytocin due to targeted disruptions of the oxytocin gene will mate, deliver their pups without apparent difficulty and display normal maternal behavior. However, they do show deficits in milk ejection and have subtle derangements in social behavior. It may be best to view oxytocin as a major facilitator of parturition and maternal behavior rather than a necessary component of these processes. Both sexes secrete oxytocin - what about its role in males? Males synthesize oxytocin in the same regions of the hypothalamus as in females, and also within the testes and perhaps other reproductive tissues. Pulses of oxytocin can be detected during ejaculation. Current evidence suggests that oxytocin is involved in facilitating sperm transport within the male reproductive system and perhaps also in the female, due to its presence in seminal fluid. It may also have effects on some aspects of male sexual behavior. Control of Oxytocin Secretion The most important stimulus for release of hypothalamic oxytocin is initiated by physical stimulation of the nipples or teats. The act of nursing or suckling is relayed within a few milliseconds to the brain via a spinal reflex arc. These signals impinge on oxytocin-secreting neurons, leading to release of oxytocin. If you want to obtain anything other than trivial amounts of milk from animals like dairy cattle, you have to stimulate oxytocin release because something like 80% of the milk is available only after ejection, and milk ejection requires oxytocin. Watch someone milk a cow, even with a machine, and what you'll see is that prior to milking, the teats and lower udder are washed gently - this tactile stimulation leads to oxytocin release and milk ejection. A number of factors can inhibit oxytocin release, among them acute stress. For example, oxytocin neurons are repressed by catecholamines, which are released from the adrenal gland in response to many types of stress, including fright. As a practical endocrine tip - don't wear a gorilla costume into a milking parlor full of cows or set off firecrackers around a mother nursing her baby. Both the production of oxytocin and response to oxytocin are modulated by circulating levels of sex steroids. The burst of oxytocin released at birth seems to be triggered in part by cervical and vaginal stimulation by the fetus, but also because of abruptly declining concentrations of progesterone. Another well-studied effect of steroid hormones is the marked increase in synthesis of uterine (myometrial) oxytocin receptors late in gestation, resulting from increasing concentrations of circulating estrogen. VAMOS AQU PARA TIROIDES Y PARATIRODES GLANDULAS Y LA FOTO 28 The Thyroid and Parathyroid Glands: Introduction and Index -----------------------------------------------------------------------Feeling run down and tired? Is your attention span poor? Do you really dislike playing in the snow? Lots of disorders can cause such complaints, but these types of symptoms are compatible with inadequate production of thyroid hormones, the primary endocrine secretion of the thyroid gland. Thyroid hormones affect three fundamental physiologic processes: cellular differentiation, growth, and metabolism. If you think about that statement for a minute, you might legitimately ask "So what else is there?" which emphasizes just how much of physiology is affected by thyroid hormones. Not many hormones can claim as diverse a set of target cells. The thyroid gland also produces another hormone called calcitonin, and the parathyroid glands secrete parathyroid hormone. Parathyroid hormone and calcitonin participate in control of calcium and phosphorus homeostasis and have significant effects on bone physiology. Functional Anatomy of the Thyroid and Parathyroid Glands -----------------------------------------------------------------------The thyroid gland is located in the neck, in close approximation to the first part of the trachea. In humans, the thyroid gland has a "butterfly" shape, with two lateral lobes that are connected by a narrow section called the isthmus. Most animals, however, have two separate glands on either side of the trachea. Thyroid glands are brownish-red in color. XXXXXXXXXXXFOTO 28 Close examination of a thyroid gland will reveal one or more small, light-colored nodules on or protruding from its surface - these are parathyroid glands (meaning "beside the thyroid"). The image to the right shows a canine thyroid gland and one attached parathyroid gland. XXXXXXXXXFOTO 29 The microscopic structure of the thyroid is quite distinctive. Thyroid epithelial cells - the cells responsible for synthesis of thyroid hormones - are arranged in spheres called thyroid follicles. Follicles are filled with colloid, a proteinaceous depot of thyroid hormone precursor (more about that later). In the low (left) and high-magnification (right) images of a cat thyroid below, follicles are cut in cross section at different levels, appearing as roughly circular forms of varying size. In standard histologic preparations such as these, colloid stains pink. In addition to thyroid epithelial cells, the thyroid gland houses one other important endocrine cell. Nestled in spaces between thyroid follicles are parafollicular or C cells, which secrete the hormone calcitonin. The structure of a parathyroid gland is distinctly different from a thyroid gland. The cells that synthesize and secrete parathyroid hormone are arranged in rather dense cords or nests around abundant capillaries. The image below shows a section of a feline parathyroid gland on the left, associated with thyroid gland (note the follicles) on the right. FotoXXXXXXXXXXXXXXX30 Chemistry of Thyroid Hormones ------------------------------------------------------------------------ Thyroid hormones are derivatives of the the amino acid tyrosine bound covalently to iodine. The two principal thyroid hormones are: * * thyroxine (known affectionately as T4 or L-3,5,3',5'-tetraiodothyronine) triiodotyronine (T3 or L-3,5,3'-triiodothyronine). FOTO XXXXXXXXXXXXXXXXXX31 As shown in the following diagram, the thyroid hormones are basically two tyrosines linked together with the critical addition of iodine at three or four positions on the aromatic rings. The number and position of the iodines is important. Several other iodinated molecules are generated that have little or no biological activity; so called "reverse T3" (3,3',5-T3) is such an example. A large majority of the thyroid hormone secreted from the thyroid gland is T4, but T3 is the considerably more active hormone. Although some T3 is also secreted, the bulk of the T3 is derived by deiodination of T4 in peripheral tissues, especially liver and kidney. Deiodination of T4 also yields reverse T3, a molecule with no known metabolic activity. Thyroid hormones are poorly soluble in water, and more than 99% of the T3 and T4 circulating in blood is bound to carrier proteins. The principle carrier of thyroid hormones is thyroxine-binding globulin, a glycoprotein synthesized in the liver. Two other carriers of import are transthyrein and albumin. Carrier proteins allow maintenance of a stable pool of thyroid hormones from which the active, free hormones are released for uptake by target cells. Synthesis and Secretion of Thyroid Hormones -----------------------------------------------------------------------Thyroid hormones are synthesized by mechanisms fundamentally different from what is seen in other endocrine systems. Thyroid follicles serve as both factory and warehouse for production of thyroid hormones. Constructing Thyroid Hormones The entire synthetic process occurs in three major steps, which are, at least in some ways, analagous to those used in the manufacture of integrated circuits (ICs): * Production and accumulation of the raw materials (in the case of ICs, a large wafer of doped silicon) * Fabrication or synthesis of the hormones on a backbone or scaffold of precursor (etching several ICs on the silicon wafer) * Release of the free hormones from the scaffold and secretion into blood (cutting individual ICs out of the larger wafer and distributing them) The recipe for making thyroid hormones calls for two principle raw materials: * Tyrosines are provided from a large glycoprotein scaffold called thyroglobulin, which is synthesized by thyroid epithelial cells and secreted into the lumen of the follicle - colloid is essentially a pool of thyroglobulin. A molecule of thyroglobulin contains 134 tyrosines, although only a handful of these are actually used to synthesize T4 and T3. * Iodine, or more accurately iodide (I-), is avidly taken up from blood by thyroid epithelial cells, which have on their outer plasma membrane a sodium-iodide symporter or "iodine trap". Once inside the cell, iodide is transported into the lumen of the follicle along with thyroglobulin. Fabrication of thyroid hormones is conducted by the enzyme thyroid peroxidase, an integral membrane protein present in the apical (colloid-facing) plasma membrane of thyroid epithelial cells. Thyroid peroxidase catalyzes two sequential reactions: 1. 2. Iodination of tyrosines on thyroglobulin (also known as "organification of iodide"). Synthesis of thyroxine (or triiodothyronine) from two iodotyrosines. Through the action of thyroid peroxidase, thyroid hormones accumulate in colloid, on the surface of thyroid epithelial cells. Remember that hormone is still tied up in molecules of thyroglobulin the task remaining is to liberate it from the scaffold and secrete free hormone into blood. XXXXXXXXXFOTO 32 Thyroid hormones are excised from their thyroglobulin scaffold by digestion in lysosomes of thyroid epithelial cells. This final act in thyroid hormone synthesis proceeds in the following steps: * Thyroid epithelial cells ingest colloid by endocytosis from their apical borders - that colloid contains thyroglobulin decorated with thyroid hormone. * Colloid-laden endosomes fuse with lysosomes, which contain hydrolytic enzymes that digest thyroglobluin, thereby liberating free thyroid hormones. Finally, free thyroid hormones apparently diffuse out of lysosomes, through the basal plasma membrane of the cell, and into blood where they quickly bind to carrier proteins for transport to target cells. XXXXXXXXXXXXXFOTO 33 Control of Thyroid Hormone Synthesis and Secretion Each of the processes described above appears to be stimulated by thyroid-stimulating hormone from the anterior pituitary gland. Binding of TSH to its receptors on thyroid epithelial cells stimulates synthesis of the iodine transporter, thyroid peroxidase and thyroglobulin. The magnitude of the TSH signal also sets the rate of endocytosis of colloid - high concentrations of TSH lead to faster rates of endocytosis, and hence, thyroid hormone release into the circulation. Conversely, when TSH levels are low, rates of thyroid hormone synthesis and release diminish. Mechanism of Action and Physiologic Effects of Thyroid Hormones -----------------------------------------------------------------------Thyroid Hormone Receptors and Mechanism of Action Receptors for thyroid hormones are intracellular DNA-binding proteins that function as hormone-responsive transcription factors, very similar conceptually to the receptors for steroid hormones. Despite being derived from an amino acid, thyroid hormones are hydrophobic in character and appear to enter cells and nuclei by diffusion through cell membranes. Once inside the nucleus, the hormone binds its receptor, and the hormone-receptor complex interacts with specific sequences of DNA in the promoters of responsive genes. The effect of receptor binding to DNA is to modulate gene expression, either by stimulating or inhibiting transcription of specific genes. For the purpose of illustration, consider one mechanism by which thyroid hormones increase the strength of contraction of the heart. Cardiac contractility depends, in part, on the relative ratio of different types of myosin proteins in cardiac muscle. Transcription of some myosin genes is stimulated by thyroid hormones, while transcription of others in inhibited. The net effect is to alter the ratio toward increased contractility. For additional details on mechanism of action and how these receptors interact with other transcription factors, examine the section Thyroid Hormone Receptors. Physiologic Effects of Thyroid Hormones It is likely that all cells in the body are targets for thyroid hormones. While not strictly necessary for life, thyroid hormones have profound effects on many "big time" physiologic processes, such as development, growth and metabolism. Many of the effects of thyroid hormone have been delineated by study of deficiency and excess states, as discussed briefly below. Metabolism: Thyroid hormones stimulate diverse metabolic activities most tissues, leading to an increase in basal metabolic rate. One consequence of this activity is to increase body heat production, which seems to result, at least in part, from increased oxygen consumption and rates of ATP hydrolysis. By way of analogy, the action of thyroid hormones is akin to blowing on a smouldering fire. A few examples of specific metabolic effects of thyroid hormones include: * Lipid metabolism: Increased thyroid hormone levels stimulate fat mobilization, leading to increased concentrations of fatty acids in plasma. They also enhance oxidation of fatty acids in many tissues. Finally, plasma concentrations of cholesterol and triglycerides are inversely correlated with thyroid hormone levels - one diagnostic indiction of hypothyroidism is increased blood cholesterol concentration. * Carbohydrate metabolism: Thyroid hormones stimulate almost all aspects of carbohydrate metabolism, including enhancement of insulin-dependent entry of glucose into cells and increased gluconeogenesis and glycogenolysis to generate free glucose. Growth: Thyroid hormones are clearly necessary for normal growth in children and young animals, as evidenced by the growth-retardation observed in thyroid deficiency. Not surprisingly, the growth-promoting effect of thyroid hormones is intimately intertwined with that of growth hormone, a clear indiction that complex physiologic processes like growth depend upon multiple endocrine controls. Development: A classical experiment in endocrinology was the demonstration that tadpoles deprived of thyroid hormone failed to undergo metamorphosis into frogs. Of critical importance in mammals is the fact that normal levels of thyroid hormone are essential to the development of the fetal and neonatal brain. Other Effects: As mentioned above, there do not seem to be organs and tissues that are not affected by thyroid hormones. A few additional, well-documented effects of thyroid hormones include: * Cardiovascular system: Thyroid hormones increases heart rate, cardiac contractility and cardiac output. They also promote vasodilation, which leads to enhanced blood flow to many organs. * Central nervous system: Both decreased and increased concentrations of thyroid hormones lead to alterations in mental state. Too little thyroid hormone, and the individual tends to feel mentally sluggish, while too much induces anxiety and nervousness. * Reproductive system: Normal reproductive behavior and physiology is dependent on having essentially normal levels of thyroid hormone. Hypothyroidism in particular is commonly associated with infertility. Thyroid Disease States Disease is associated with both inadequate production and overproduction of thyroid hormones. Both types of disease are relatively common afflictions of man and animals. Hypothyroidism is the result from any condition that results in thyroid hormone deficiency. Two well-known examples include: * Iodine deficiency: Iodide is absolutely necessary for production of thyroid hormones; without adequate iodine intake, thyroid hormones cannot be synthesized. Historically, this problem was seen particularly in areas with iodine-deficient soils, and frank iodine deficiency has been virtually eliminated by iodine supplementation of salt. * Primary thyroid disease: Inflammatory diseases of the thyroid that destroy parts of the gland are clearly an important cause of hypothyroidism. Common symptoms of hypothyroidism arising after early childhood include lethargy, fatigue, coldintolerance, weakness, hair loss and reproductive failure. If these signs are severe, the clinical condition is called myxedema. In the case of iodide deficiency, the thyroid becomes inordinantly large and is called a goiter. The most severe and devestating form of hypothyroidism is seen in young children with congenital thyroid deficiency. If that condition is not corrected by supplemental therapy soon after birth, the child will suffer from cretinism, a form of irreversible growth and mental retardation. Most cases of hypothyroidism are readily treated by oral administration of synthetic thyroid hormone. In times past, consumption of dessicated animal thyroid gland was used for the same purpose. Hyperthyroidism results from secretion of thyroid hormones. In most species, this condition is less common than hypothyroidism. In humans the most common form of hyperthyroidism is Graves disease, an immune disease in which autoantibodies bind to and activate the thyroidstimulating hormone receptor, leading to continual stimulation of thyroid hormone synthesis. Another interesting, but rare cause of hyperthyroidism is so-called hamburger thyrotoxicosis. Common signs of hyperthyroidism are basically the opposite of those seen in hypothyroidism, and include nervousness, insomnia, high heart rate, eye disease and anxiety. Control of Thyroid Hormone Synthesis and Secretion -----------------------------------------------------------------------The chief stimulator of thyroid hormone synthesis is thyroid-stimulating hormone from the anterior pituitary. Binding of TSH to receptors on thyroid epithelial cells seems to enhance all of the processes necessary for synthesis of thyroid hormones, including synthesis of the iodide transporter, thyroid peroxidase and thyroglobulin. XXXXXXXXXXXXXXXXXXXXXFOTO 34 The magnitude of the TSH signal also sets the rate of endocytosis of colloid - high concentrations of TSH lead to faster rates of endocytosis, and hence, thyroid hormone release into the circulation. Conversely, when TSH levels are low, rates of thyroid hormone synthesis and release diminish. The thyroid gland is part of the hypothalamic-pituitary-thyroid axis, and control of thyroid hormone secretion is exerted by classical negative feedback, as depicted in the diagram. Thyroid-releasing hormone (TRH) from the hypothalamus stimulates TSH from the pituitary, which stimulates thyroid hormone release. As blood concentrations of thyroid hormones increase, they inhibit both TSH and TRH, leading to "shutdown" of thyroid epithelial cells. Later, when blood levels of thyroid hormone have decayed, the negative feedback signal fades, and the system wakes up again. A number of other factors have been shown to influence thyroid hormone secretion. In rodents and young children, exposure to a cold environment triggers TRH secretion, leading to enhanced thyroid hormone release. This makes sense considering the known ability of thyroid hormones to spark body heat production. Calcitonin -----------------------------------------------------------------------Calcitonin is a hormone known to participate in calcium and phosphorus metabolism. A major source of calcitonin is from the parafollicular or C cells in the thyroid gland, but it is also synthesized in a wide variety of other tissues, including the lung and intestinal tract. Calcitonin is a 32 amino acid peptide cleaved from a larger prohormone. It contains a single disulfide bond, which causes the amino terminus to assume the shape of a ring. The calcitonin receptor has been cloned and shown to be a member of the seven-transmembrane, G proteincoupled receptor family. Physiologic Effects of Calcitonin A large and diverse set of effects has been attributed to calcitonin, but in many cases, these were seen in response to pharmacologic doses of the hormone, and their physiologic relevance is suspect. It seems clear however, that calcitonin plays a role in calcium and phosphorus metabolism. In particular, calcitonin has the ability to decrease blood calcium levels at least in part by effects on two well-studied target organs: * Bone: Calcitonin suppresses resorption of bone by inhibiting the activity of osteoclasts, a cell type that "digests" bone matrix, releasing calcium and phosphorus into blood. * Kidney: Calcium and phosphorus are prevented from being lost in urine by reabsorption in the kidney tubules. Calcitonin inhibits tubular reabsorption of these two ions, leading to increased rates of their loss in urine. Despite the effects on calcium described above, it seems that calcitonin has at best a minor role in regulating blood concentrations of calcium. One interesting piece of evidence to support this statement is that humans with chronically increased (medullary thyroid cancer) or decreased (surgical removal of the thyroid gland) levels of calcitonin in blood do not show alterations from normal in serum calcium. Addition information on calcitonin and calcium balance can be found in the section Endocrine Control of Calcium Homeostasis. Control of Calcitonin Secretion Factors that regulate calcitonin synthesis and secretion are very poorly understood. A number of other hormones have been shown to stimulate calcitonin release in certain situations, and nervous controls also have been demonstrated. Disease States A large number of diseases are associated with abnormally increased or decreased levels of calcitonin, but pathologic effects of abnormal calcitonin secretion per se are not generally recognized. There are several therapeutic uses for calcitonin. It is used to treat hypercalcemia resulting from a number of causes, and has been a valuable therapy for Paget disease, which is a disorder in bone remodeling. Calcitonin also appears to be a valuable aid in the management of certain types of osteoporosis. Parathyroid Hormone -----------------------------------------------------------------------Parathyroid hormone is the most important endocrine regulator of calcium and phosphorus concentration in extracellular fluid. This hormone is secreted from cells of the parathyroid glands and finds its major cells in bone and kidney. Another hormone, parathyroid hormonerelated protein, binds to the same receptor as parathyroid hormone and has major effects on development. Like most other protein hormones, parathyroid hormone is synthesized as a preprohormone. After intracellular processing, the mature hormone is packaged within the Golgi into secretory vesicles, the secreted into blood by exocytosis. Parathyroid hormone is secreted as a linear protein of 84 amino acids. Physiologic Effects of Parathyroid Hormone Writing a job description for parathyroid hormone is straightforward: if calcium ion concentrations in extracellular fluid fall below normal, bring them back within the normal range. In conjunction with increasing calcium concentration, the concentration of phosphate ion in blood is reduced. Parathyroid hormone accomplishes its job by stimulating at least three processes: * Mobilization of calcium from bone: Although the mechanisms remain obscure, a welldocumented effect of parathyroid hormone is to stimulate osteoclasts to reabsorb bone mineral, liberating calcium into blood. * Enhancing absorption of calcium from the small intestine: Facilitating calcium absorption from the small intestine would clearly serve to elevate blood levels of calcium. Parathyroid hormone clearly stimulates this process, but indirectly by stimulating production of the active form of vitamin D in the kidney. Vitamin D induces synthesis of a calcium-binding protein in intestinal epithelial cells that facilitates efficient absorption of calcium into blood. * Suppression of calcium loss in urine: In addition to stimulating fluxes of calcium into blood from bone and intestine, parathyroid hormone puts a brake on excretion of calcium in urine, thus conserving calcium in blood. This effect is mediated by stimulating tubular reabsorption of calcium. Another effect of parathyroid hormone on the kidney is to stimulate loss of phosphate ions in urine. XXXXXXXXXXFOTO 35 Addition information on how parathyroid hormone and vitamin D control calcium balance can be found in the section Endocrine Control of Calcium Homeostasis. Control of Parathyroid Hormone Secretion XXXXXXXXXXXFOTO 36 Parathyroid hormone is released in response to low extracellular concentrations of free calcium. Changes in blood phosphate concentration can be associated with changes in parathyroid hormone secretion, but this appears to be an indirect effect and phosphate per se is not a significant regulator of this hormone. When calcium concentrations fall below the normal range, there is a steep increase in secretion of parathyroid hormone. Low levels of the hormone are secreted even when blood calcium levels are high. The figure to the right depicts parathyroid hormone release from cells cultured in vitro in differing concentrations of calcium. The parathyroid cell monitors extracellular free calcium concentration via an integral membrane protein that functions as a calcium-sensing receptor. Disease States Both increased and decreased secretion of parathyroid hormone are recognized as causes of serious disease in man and animals. Excessive secretion of parathyroid hormone is seen in two forms: * Primary hyperparathyroidism is the result of parathyroid gland disease, most commonly due to a parathyroid tumor (adenoma) which secretes the hormone without proper regulation. Common manifestations of this disorder are chronic elevations of blood calcium concentration (hypercalcemia), kidney stones and decalcification of bone. * Secondary hyperparathyroidism is the situation where disease outside of the parathyroid gland leads to excessive secretion of parathyroid hormone. A common cause of this disorder is kidney disease - if the kidneys are unable to reabsorb calcium, blood calcium levels will fall, stimulating continual secretion of parathyroid hormone to maintain normal calcium levels in blood. Secondary hyperparathyroidism can also result from inadequate nutrition - for example, diets that are deficient in calcium or vitamin D, or which contain excessive phosphorus (e.g. all meat diets for carnivores). A prominent effect of secondary hyperparathyroidism is decalcification of bone, leading to pathologic fractures or "rubber bones". Chronic secretion or continuous infusion of parathyroid hormone clearly leads to decalcification of bone and loss of bone mass. However, in certain situations, treatment with parathyroid hormone can actually stimulate an increase in bone mass and bone strength. This seemingly paradoxical effect occurs when the hormone is administered by once daily injection, and such treatment is under evaluation as a therapy for diseases such as osteoporosis. Inadequate production of parathyroid hormone - hypoparathyroidism - typically results in decreased concentrations of calcium and increased concentrations of phosphorus in blood. Common causes of this disorder include surgical removal of the parathyroid glands and disease processes that lead to destruction of parathyroid glands. The resulting hypocalcemia often leads to tetany and convulsions, and can be acutely life-threatening. Treatment focuses on restoring normal blood calcium concentrations by calcium infusions, oral calcium supplements and vitamin D therapy. Thyroid Hormone Receptors -----------------------------------------------------------------------Receptors for thyroid hormones are members of a large family of nuclear receptors that include those of the steroid hormones. They function as hormone-activated transcription factors and thereby act by modulating gene expression. In contrast to steroid hormone receptors, thyroid hormone receptors bind DNA in the absence of hormone, usually leading to transcriptional repression. Hormone binding is associated with a conformational change in the receptor that causes it to function as a transcriptional activator. XXXXXXXXXXXXFOTOT 37 Receptor Structure Mammalian thyroid hormone receptors are encoded by two genes, designated alpha and beta. Further, the primary transcript for each gene can be alternatively spliced, generating different alpha and beta receptor isoforms. Currently, four different thyroid hormone receptors are recognized: alpha-1, alpha-2, beta-1 and beta-2. Like other members of the nuclear receptor superfamily, thyroid hormone receptors encapsulate three functional domains: * A transactivation domain at the amino terminus that interacts with other transcription factors to form complexes that repress or activate transcription. There is considerable divergence in sequence of the transactivation domains of alpha and beta isoforms and between the two beta isoforms of the receptor. * A DNA-binding domain that binds to sequences of promoter DNA known as hormone response elements. * A ligand-binding and dimerization domain at the carboxy-terminus. As depicted in the figure below, the DNA-binding domains of the different receptor isoforms are very similar, but there is considerable divergence among transactivation and ligand-binding domains. Most notably, the alpha-2 isoform has a unique carboxy-terminus and does not bind triiodothyronine (T3). The different forms of thyroid receptors have patterns of expression that vary by tissue and by developmental stage. For example, almost all tissues express the alpha-1, alpha-2 and beta-1 isoforms, but beta-2 is synthesized almost exclusively in hypothalamus, anterior pituitary and developing ear. Receptor alpha-1 is the first isoform expressed in the conceptus, and there is a profound increase in expression of beta receptors in brain shortly after birth. Interestingly, the beta receptor preferentially activates expression from several genes known to be important in brain development (e.g. myelin basic protein), and upregulation of this particular receptor may thus be critical to the well known effects of thyroid hormones on development of the fetal and neonatal brain. The presence of multiple forms of the thyroid hormone receptor, with tissue and stagedependent differences in their expression, suggests an extraordinary level of complexity in the physiologic effects of thyroid hormone. Interaction of Thyroid Hormone Receptors with DNA Thyroid hormone receptors bind to short, repeated sequences of DNA called thyroid or T3 response elements (TREs), a type of hormone response element. A TRE is composed of two AGGTCA "half sites" separated by four nucleotides. The half sites of a TRE can be arranged as direct repeats, pallindromes or inverted repeats. The DNA-binding domain of the receptor contains two sets of four cysteine residues, and each set chelates a zinc ion, forming loops known as "zinc fingers". A part of the first zinc finger interacts directly with nucleotides in the major groove of TRE DNA, while residues in the second finger interact with nucleotides in the minor groove of the TRE. Thus, the zinc fingers mediate specificity in binding to TREs. Thyroid hormone receptors can bind to a TRE as monomers, as homodimers or as heterodimers with the retinoid X receptor (RXR), another member of the nuclear receptor superfamily that binds 9-cis retinoic acid. The heterodimer affords the highest affinity binding, and is thought to represent the major functional form of the receptor. Thyroid hormone receptors bind to TRE DNA regardless of whether they are occupied by T3. However, the biological effects of TRE binding by the unoccupied versus the occupied receptor are dramatically different. In general, binding of thyroid hormone receptor alone to DNA leads to repression of transcription, whereas binding of the thyroid hormone-receptor complex activates transcription. Ligand-free state: The transactivation domain of the T3-free receptor, as a heterodimer with RXR, assumes a conformation that promotes interaction with a group of transcriptional corepressor molecules. A part of this corepressor complex has histone deacetylase activity (HDA), which is associated with formation of a compact, "turned-off" conformation of chromatin. The net effect of recruiting these types of transcription factors is to repress transcription from affected genes. Ligand-bound state: Binding of T3 to its receptor induces a conformational change in the receptor that makes it incompetent to bind the corepressor complex, but competent to bind a group of coactivator proteins. The coactivator complex contains histone transacetylase (HAT) activity, which imposes an open configuration on adjacent chromatin. The coactivator complex associated with the T3-bound receptor functions to activate transcription from linked genes. A growing number of specific proteins have been identified as members of the corepressor and coactivator complexes described. It should also be mentioned that there are several exceptions to the scheme described above. As mentioned, the alpha-2 receptor is unable to bind T3 and acts as similarly to a dominant-negative mutant of the receptor, but its carboxy-terminus can be differentially phosphorylated, which affects DNA binding and dimerization. Also, the beta-2 isoform apparently fails to function as a repressor in the absence of T3. Disorders of Thyroid Hormone Receptors A number of humans with a syndrome of thyroid hormone resistance have been identified, and found to have mutations in the receptor beta gene which abolish ligand binding. Clinicially, such individuals show a type of hypothyroidism characterized by goiter, elevated serum concentrations of T3 and thyroxine and normal or elevated serum concentrations of TSH. More than half of affected children show attention-deficit disorder, which is intriguing considering the role of thyroid hormones in brain development. In most affected families, this disorder is transmitted as a dominant trait, which suggests that the mutant receptors act in a dominant negative manner. Mice with targeted deletions in thyroid receptor genes have provided additional understanding of the possible roles of different forms of thyroid hormone receptors. Knockout mice that are unable to produce the alpha-1 receptor showed subnormal body temperature and mild abnormalities in cardiac function. Other mice which lacked expression of both alpha isoforms were severely hypothyroid and died within the first few weeks of life. Mice with disruptions of the entire beta gene exhibited elevated TSH levels and deafness, while mice with mutations that disrupted only beta-2 expression had elevated TSH, but normal hearing. Such experiments are beginning to allow determination of which functions of the different receptor isoforms are redundant and which are not. Inactivating mutations in thyroid hormone receptors do not produce a syndrome analogous to the lack of thyroid hormones. This is the case even in mice with targeted deletions in both alpha and beta receptor genes. The most likely explanation for the relative mild effects of receptor deficiency is that responsive genes are left in a "neutral" state, rather than being chronically suppressed as happens with hormone deficiency. Thyroid Hormones: Pregnancy and Fetal Development -----------------------------------------------------------------------Thyroid hormones are critical for development of the fetal and neonatal brain, as well as for many other aspects of fetal growth. Hypothyroidism in either the mother or fetus frequently results in fetal disease; in humans, this includes a high incidence of mental retardation. Maternal Thyroid Function During Pregnancy Normal pregnancy entails substantial changes in thyroid function in all animals. These phenomena have been studied most extensively in humans, but probably are similar in all mammals. Major alterations in the thyroid system during pregnancy include: * Increased blood concentrations of T4-binding globulin: TBG is one of several proteins that transport thyroid hormones in blood, and has the highest affinity for T4 (thyroxine) of the group. Estrogens stimulate expression of TBG in liver, and the normal rise in estrogen during pregnancy induces roughly a doubling in serum TBG concentratrations. Increased levels of TBG lead to lowered free T4 concentrations, which results in elevated TSH secretion by the pituitary and, consequently, enhanced production and secretion of thyroid hormones. The net effect of elevated TBG synthesis is to force a new equilibrium between free and bound thyroid hormones and thus a significant increase in total T4 and T3 levels. The increased demand for thyroid hormones is reached by about 20 weeks of gestation and persists until term. * Increased demand for iodine: This results from a significant pregnancy-associated increase in iodide clearance by the kidney (due to increased glomerular filtration rate), and siphoning of maternal iodide by the fetus. The World Health Organization recommends increasing iodine intake from the standard 100 to 150 ug/day to at least 200 ug/day during pregnancy. Thyroid stimulation by chorionic gonadotropin: The placentae of humans and other primates secrete huge amounts of a hormone called chorionic gonadotropin (in the case of humans, human chorionic gonadotropin or hCG) which is very closely related to luteinizing hormone. TSH and hCG are similar enough that hCG can bind and transduce signalling from the TSH receptor on thyroid epithelial cells. XXXXXXXXXXXXXFOTO 38 Toward the end of the first trimester of pregnancy in humans, when hCG levels are highest, a significant fraction of the thyroid-stimulating activity is from hCG. During this time, blood levels of TSH often are suppressed, as depicted in the figure to the right. The thyroid-stimulating activity of hCG actually causes some women to develop transient hyperthyroidism. One would predict that a similar phenomenon might occur in horses, who also secrete a placental gonadotropin which is actually equine luteinizing hormone. The net effect of pregnancy is an increased demand on the thyroid gland. In the normal individuals, this does not appear to represent much of a load to the thyroid gland, but in females with subclinical hypothyroidism, the extra demands of pregancy can precipitate clinicial disease. Thyroid Hormones and Fetal Brain Development In 1888 the Clinical Society of London issued a report underlining the importance of normal thyroid function on development of the brain. Since that time, numerous studies with rats, sheep and humans have reinforced this concept, usually by study of the effects of fetal and/or maternal thyroid deficiency. Phenomenologically, thyroid hormones appear to have their most profound effects on the terminal stages of brain differentiation, including synaptogenesis, growth of dendrites and axons, myelination and neuronal migration. Thyroid hormones act by binding to nuclear receptors and modulating transcription of responsive genes. Thyroid hormone receptors are widely distributed in the fetal brain, and present prior to the time the fetus is able to synthesize thyroid hormones. It has proven surprisingly difficult to identify the molecular targets for thyroid hormone action in the developing brain, but some progress has been made. For example, the promoter of the myelin basic protein gene is directly responsive to thyroid hormones and contains the expected hormone response element. This fits with the observation that induced hypothyroidism in rats leads to diminished synthesis of mRNAs for several myelin-associated proteins. It seems clear that there is a great deal more to learn about the molecular mechanisms by which thyroid hormones support normal development of the brain. Thyroid Deficiency in the Fetus and Neonate The fetus has two potential sources of thyroid hormones - it's own thyroid and the thyroid of it's mother. Human fetuses acquire the ability to synthesize thyroid hormones at 10 to 12 weeks of gestation, and fetuses from other species at developmentally similar times. Current evidence from several species indicates that there is substantial transfer of maternal thyroid hormones across the placenta. Additionally, the placenta contains deiodinases that can convert T4 to T3. There are three types or combinations of thyroid deficiency states known to affect fetal development: * Isolated maternal hypothyroidism: Overt maternal hypothyroidism typically is not a significant cause of fetal disease because it usually is associated with infertility. When pregnancy does occur, there is increased risk of intrauterine fetal death and gestational hypertension. Subclincial hypothyroidism is increasingly being recognized as a cause of developmental disease this is a rather scary situation. Several investigators have found that mild maternal hypothyroidism, diagnosed only retrospectively from banked serum, may adversely affect the fetus, leading in children to such effects as slightly lower performance on IQ tests and difficulties with schoolwork. The most common cause of subclinical hypothyroidism is autoimmune disease, and it is known that anti-thyroid antibodies cross the human placenta. Thus, the cause of this disorder may be a passive immune attack on the fetal thyroid gland. * Isolated fetal hypothyroidism: This condition is also known as sporadic congenital hypothyroidism. It is due to failure of the fetal thyroid gland to produce adequate amounts of thyroid hormone. Most children with this disorder are normal at birth, because maternal thyroid hormones are transported across the placenta during gestation. What is absolutely critical is to identify and treat this condition very shortly after birth. If treatment is not instituted quickly, the child will become permanently mentally and growth retarded - a disorder called cretinism. This problem has largely disappeared in the US and many other countries due to large scale screening programs to detect hypothyroid infants. * Iodine deficiency - Combined maternal and fetal hypothyroidism: Iodine deficiency is, by a large margin, the most common preventable cause of mental retardation in the world. Without adequate maternal iodine intake, both the fetus and mother are hypothyroid, and if supplemental iodine is not provided, the child may well develop cretinism, with mental retardation, deaf-mutism and spasticity. The World Health Organization estimated in 1990 that 20 million people had some degree of brain damage due to iodine deficiency experienced in fetal life. Endemic iodine deficiency remains a substantial public health problem in many parts of the world, including many areas in Europe, Asia, Africa and South America. In areas of severe deficiency, a large fraction of the adult population may show goiters. In such settings, overt cretinism may occur in 5 to 10 percent of offspring, and perhaps five times that many children will have mild mental retardation. This is a serious, tragic and, most importantly, a preventable problem. The fetus of an iodine-deficient mother can be successfully treated if iodine supplementation is given during the first or second trimester. Treatment during the third trimester or after birth will not prevent the mental defects. Iodine deficiency can also be a sigificant problem in animal populations. The most common manifestation in sheep, cattle, pigs and horses is a high incidence of stillbirths and birth of small, weak offspring. Hyperthyroidism in Pregnancy Gestational hyperthyroidism is associated with increased risk of several adverse outcomes, including preeclampisa, premature labor, fetal or perinatal death and low birth weight. In humans, hyperthyroidism usually is the result of Grave's disease, which involves development of autoantibodies against the TSH receptor that stimulate the thyroid gland. Hamburger Thyrotoxicosis -----------------------------------------------------------------------Thyroid hormones are orally active, which means that consumption of thyroid gland tissue can cause thyrotoxicosis, a type of hyperthyroidism. Several outbreaks of thyrotoxicosis have been attributed to a practice, now banned in the US, called "gullet trimming", where meat in the neck region of slaughtered animals is ground into hamburger. Because thyroid glands are reddish in color and located in the neck, it's not unusual for gullet trimmers to get thyroid glands into hamburger or sausage. People, and presumably pets, that eat such hamburger can get dose of thyroid hormone sufficient to induce disease. A report by Hedberg and colleagues (1987) on this topic is one of several in the literature. They described an outbreak of thyrotoxicosis in Minnesota and South Dakota that was traced to thyroid-contaminated hamburger. A total of 121 cases were identified in nine counties, with the highest incidence in the county having the offending slaughter plant. The patients complained of sleeplessness, nervousness, headache, fatique, excessive sweating and weight loss. The graph below shows serum concentrations of thyroxine and thyroid-stimulating hormone in a volunteer that consumed a well-cooked, 227 g hamburger (admittedly, a large meal) prepared from the contaminated meat. Note how TSH levels were suppressed during the time when thyroxine (T4) concentrations were elevated. XXXXXXXXXFOTO 39 The Sodium-Iodide Symporter -----------------------------------------------------------------------Physiologic and Pathologic Significance The ability of the thyroid gland to transport and concentrate iodide from blood is absolutely necessary for the synthesis of thyroid hormones. The key player in this process is the sodiumiodide symporter, an integral membrane protein that resides in the basolateral membrane of thyroid epithelial cells. Considering critical role of iodine trapping in thyroid function, it is not surprising that abnormalities in expression or function of the symporter can lead to thyroid diesase. Two such situations have been identified in humans: * Inactivating mutations in the symporter gene result in congenital hypothyroidism. In several patients with this disorder, specific missense mutations in the symporter mRNA have been characterized. * Autoantibodies to the symporter protein adversely affect iodide transport. A substantial number of patients with autoimmune (Hasimoto's) thyroiditis have anti-symporter antibodies, and application of these antibodies to cultured cells expressing the symporter inhibits iodide uptake. The sodium-iodide symporter cannot distinguish between normal and radioactive iodide, thus providing a useful exploit for diagnosis and treatment of certain thyroid disease. Small amounts of radioactive iodine injected into patients are rapidly concentrated in the thyroid, providing a means to image the thyroid for detection of tumors and other abnormalities. Administration of higher doses of radioiodine is widely used for treatment of hyperthyroidism and some types of thyroid cancer; in this case the radioactivity is concentrated rather precisely in the tissue requiring destruction. Structure and Function As its name indicates, the sodium-iodide symporter simultaneously transports both Na+ and I- ions from extracellular fluid (i.e. blood) into the thryoid epithelial cell. This process is an example of secondard active transport. Energy is provided by the electrochemical gradient of sodium across the cell membrane; the low intracellular concentration of sodium is maintained by sodium pumps. FFFFFFFFFFFFFFFOTO 40 Recently, cDNAs for the rat and human sodium-iodide transporter have been cloned. Analysis of their deduced protein sequence indicates that the symporter has 13 membrane-spanning domains. The human and rat proteins are 643 and 618 amino acids in length and both contain N-linked glycosylation sites. Although the symporter appears to be glycosylated, those modifications are appearently not required for full activity. The functional receptor may be a multimer. Regulation of Expression The sodium-iodide symporter is most highly expressed in thyroid epithelial cells. Low levels of expression can be detected in mammary gland, salivary gland, stomach and colon, but none of these tissues is known to organify iodide. The presence of the symporter in mammary gland leads to secretion of iodine in milk, which is probably important for thyroid function in neonatal animals. The most important stimulator of symporter gene and protein expression is thyroid-stimulating hormone, similar to what is observed with other important thyroid proteins such as thyroglobulin and thyroid peroxidase. The human sodium-iodide symporter gene has 15 exons. It has a basal promoter region that extends roughly 500 bp upstream from the transcription start site and thyroid-specific enhancer elements that span positions of approximately -2200 to -2500. Parathyroid Hormone-Related Protein -----------------------------------------------------------------------Parathyroid hormone-related protein (PTHrP) is actually a family of protein hormones produced by most if not all tissues in the body. A segment of PHRH is closely related to parathyroid hormone, and hence its name, but PHRH peptides have a much broader spectrum of effects. Parathyroid hormone and some of the PTHrP peptides bind to the same receptor, but PTHrP peptides also bind to several other receptors. PHRH was discovered as a protein secreted by certain tumors that caused hypercalcemia (elevated blood calcium levels) in affected patients. It was soon shown that the uncontrolled secretion of PHRH by many tumor cells induces hypercalcemia by stimulating resorption of calcium from bone and suppressing calcium loss in urine, similar to what is seen with hyperparathyroidism. However, it quickly become apparent that PHRH had many activities not seen with parathyroid hormone. Hormone Structures, Receptors and Sources PTHrP is encoded by a single gene that is highly conserved among species. It should probably be described as a polyhormone, because a family of peptide hormones are generated by alternative splicing of the primary transcript and through use of alternative post-translational cleavage sites. To make matters even more complex, some cells appear to use alternative translational initiation codons to produce forms of the protein that are targeted either for secretion or nuclear localization. The figure below shows one of the characterized processing patterns of the PTHrP preprohormone, in this case yielding 3 bioactive peptides. The diverse activities of PTHrP result not only from processing of the precursor into multiple hormones, but from use of multiple receptors. It is clear that amino-terminal peptides of PTHrP share a receptor with parathyroid hormone, but they also bind to a type of receptor in some tissues that does not bind parathyroid hormone. Moreover, it is almost certain that the midregion and osteostatin peptides bind other, unique receptors. In addition to the secreted forms of this hormone, there is considerable evidence that a form of PTHrP is generated in some cells that is not secreted and, via nuclear targeting sequences, is translocated to the nucleus, where it affects nuclear function. The consequences of this "intracrine" mode of action are not yet well characterized, but may modulate such important activities as programmed cell death. PTHrP is secreted from a large and diverse set of cells, and during both fetal and postnatal life. Among tissues known to secrete this hormone are several types of epithelium, mesenchyme, vascular smooth muscle and central nervous system. Although PTHrP is found in serum, a majority of its activity appears to reflect paracrine signaling. Physiologic Effects of Parathyroid Hormone-Related Protein One thing to recognize about PTHrP is that its name is inadequate to describe its activities. Like parathyroid hormone, some of the effects of PTHrP result from its effects on transepithelial fluxes of calcium, but many of its actions have nothing to do with calcium homeostasis. Most prominently, PTHrP peptides exert significant control over the proliferation, differentiation and death of many cell types. They also play a major role in development of several tissues and organs. Much of our understanding of the biologic effects of PTHrP comes from experiments with genetically-altered mice. Mice with targeted deletions in the PTHrP gene (knockout mice), mice that overexpress PTHrP in specific tissues (transgenic mice), and crosses between knockout and transgenic mice have been critical in delineating many effects of this hormone. Humans with mutations in the PTHrP gene or the parathyroid receptor have also played a role in confirming the activity of PTHrP. Some of the physiologic effects of PTHrP garnered from these studies are described here: Cartilage and Bone Development: Mice with homozygous inactivation of the PTHrP gene die at birth, if not earlier. They manifest severe chondrodysplasia and premature epiphyseal closure, reflecting a developmental defect in proliferation and differentiation of cartilage. These and other types of studies indicate that PTHrP stimulates the proliferation of chondrocytes and suppresses their terminal differentiation. These effects of PTHrP appear due to interaction of the PTH-like peptide with the parathyroid hormone receptor. Mammary Development and Lactation: The mammary glands of female mice with homozygous inactivation of the PTHrP gene fail to develop, except for the earliest stages. Development of the mammary gland depends upon a complex interaction between epithelial and mesenchymal cells that apparently requires PTHrP. In normal animals, mammary epithelial cells secrete large amounts of PTHrP, which suggests a role of this hormone in adapting maternal metabolism to the calcium demands of lactation. Placental Transfer of Calcium: The "midregion" peptide of PTHrP (see above) has been shown to control the normal maternal-to-fetal pumping of calcium across the placenta. In the absence of fetal PTHrP, this gradient is not established. Smooth Muscle Functioning: PTHrP is secreted from smooth muscle in many organs, usually in response to stretching. It acts to relax smooth muscle, thereby serving, among other things, as a vasodilating hormone. Transgenic mice that express PTHrP in vascular smooth muscle manifest hypotension. PTHrP may also have effects on contraction of muscle in the bladder, uterus and heart. Other Effects: PTHrP is highly expressed in skin. Transgenic mice that overexpress PTHrP in skin show alopecia, and treatment of mice with a PTHrP antagonist leads to increased numbers of hair follicles and a shaggy appearance. Another interesting defect in PTHrP-knockout mice is that, although their teeth develop normally, they fail to erupt. Finally, both PTHrP and its receptors are widely expressed in the central nervous system, and appear to influence neuronal survival by several mechanisms. It should be clear from the above examples that PTHrP hormones have profound effects on a large number of physiologic processes. Ongoing research on this polyhormone is certain to reveal additional effects in this already complex system. Parathyroid Hormone Receptors -----------------------------------------------------------------------Parathyroid hormone and its cousin parathyroid hormone-related protein (PTHrP) are critical controllers of calcium and phosphorus balance. The receptors for these two hormones are under close scrutiny, because such understanding may facilitate development of antagonists for treatment of a number of important diseases, including osteoporosis and hypercalcemia associated with some types of cancer. Two receptors have been identified that bind parathyroid hormone, one of which also binds PTHrP: Type 1 parathyroid hormone receptor: Binds both parathyroid hormone and amino-terminal peptides of PTHrP. This molecule is a G protein-coupled receptor with seven transmembrane segments. The extracellular domain has six cysteine residues. Binding of ligand to this receptor activates both adenylyl cyclase and phospholipase C systems, generating protein kinase A and protein kinase C signals, respectively. The cyclic AMP/protein kinase A pathway is predominant. As might be expected from the actions of parathyroid hormone, the mRNA encoding the type 1 receptor is most abundant in bone (especially in chondrocytes at growth plates) and kidney. The mRNA is also expressed at lower levels in many other tissues, probably reflecting its use as a receptor for PTHrP. Type 2 parathyroid hormone receptor: Binds parathyroid hormone, but shows very low affinity for PTHrP. This molecule is expressed in only a few tissues, and its structure and physiologic significance are poorly characterized. Like the type 1 receptor, it is coupled to adenylyl cyclase and ligand binding induces a rise in intracellular concentration of cyclic AMP. Mutations in the type 1 receptor have been associated with rare human diseases. Jansen's metaphyseal chondroplasia is a syndrome of short limbed dwarfism resulting from a mutation that constitutively activates the receptor. Blomstrand's chondroplasia results from an inactivating mutation in the receptor gene, leading to a disease of early death with defective bone maturation, very similar to mice with targeted deletions of the PTHrP gene. The Extracellular Calcium-Sensing Receptor -----------------------------------------------------------------------Maintaining tight control over the concentration of calcium in blood and extracellular fluid is a critical task. It stands to reason that a calcium sensor would evolve as a component of the system responsible for calcium homeostasis. Considering its involvement in modulating so many physiologic processes, calcium itself can be thought of as a type of hormone, and the calcium sensor as its receptor. The DNA sequence encoding the extracellular calcium sensor was originally isolated from bovine parathyroid gland. Since then, corresponding sequences have been isolated from a broad range of species, enabling serious study of this intriguing membrane protein. The calcium-sensing receptor is a member of the G protein-coupled receptor family. Like other family members, it contains seven hydrophobic helices that anchor it in the plasma membrane. The large (~600 amino acids) extracellular domain is known to be critical to interactions with extracellular calcium. The receptor also has a rather large (~200 amino acids) cytosolic tail. These features are depicted in the figure to the right; the red highlights on the intracellular domain correspond to potential protein kinase phosphorylation sites. Activation of the calcium sensor has two major signal-transducing effects: * Activation of phospholipase C, which leads to generation of the second messengers diacylglycerol and inositol trisphosphate. * Inhibition of adenylate cyclase, which suppresses intracellular concentration of cyclic AMP. The sensor can also activate the mitogen-activated protein kinase pathway, suggesting an ability to influence nuclear function. The calcium sensor is expressed in a broad range of cells, including parathyroid cells and C cells in the thyroid gland, indicating its involvement in controlling the synthesis and secretion of parathyroid hormone and calcitonin. Functional studies and investigation of animals with mutations in the calcium sensor gene have confirmed that the calcium sensor directly affects secretion of these two hormones. The calcium sensor is also expressed in several cell types in the kidney, osteoblasts, a variety of hematopoietic cells in bone marrow, and in the gastrointestinal mucosa. Strangely, it is also present in the squamous epithelial cells of the esophagus. Such a broad distribution of expression supports that concept that calcium, acting as a hormone, has direct effects on the function of many cell types. Understanding the role of the calcium sensor in calcium homeostasis has benefitted greatly by the study of mutations in the human gene encoding this receptor: * Inactivating mutations: A large number of different mutations in the calcium sensor gene have been identified in patients affected by a type of calcium resistance known as familial hypocalciuric hypercalcemia. The different mutations result in a spectrum of calcium sensor dynfunctions, ranging from total inactivation to a moderate decrease in affinity of the receptor for calcium. A prominent clinical consequence of such mutations is an abnormal set point or sensitivity of the parathyroid gland to blood calcium concentration. Abnormalities in renal excretion of calcium are also observed. * Activating mutations: Certain types of mutations lead to a calcium sensor with an elevated sensitivity to calcium. The clinical consequence of such mutations is familial hypercalciuric hypocalcemia - basically the opposite of what is seen with inactivating mutations in the sensor gene. High blood calcium affects the normal calcium sensor to suppress parathyroid hormone secretion. If the calcium sensor is constitutively more active than, a type of hypoparathyroidism results. Further study of the extracellular calcium-sensing receptor will undoubtedly shed further light on the intricacies of how calcium homeostasis is regulated, and may provide new opportunities for treatment of diseases of calcium metabolism. The Adrenal Gland: Introduction and Index -----------------------------------------------------------------------When you think about the adrenal glands, you should think about stress. Stress can take many forms: taking an examination, recovering from a broken bone, running away from an invading army, or maintaining proper levels of energy substrates in the face of even mild starvation. For human males, there is even considerable stress associated with shopping. The adrenal produces three major classes of hormones, each of which aid in dealing with the multitude of small and large stresses faced by animals and people almost daily. There is no doubt that at least two of these groups - glucocorticoids and mineralocorticoids - are necessary for life. Functional Anatomy of the Adrenal Gland -----------------------------------------------------------------------XXXXXXXXFOTO 41 The two adrenal glands are located immediately anterior to the kidneys, encased in a connective tissue capsule and usually partially buried in an island of fat. Like the kidneys, the adrenal glands lie beneath the peritoneum (i.e. they are retroperitoneal). The exact location relative to the kidney and the shape of the adrenal gland vary among species. Inspection of a mammalian adrenal gland that has been sectioned reveals two distinct regions, as demonstrated to the right with a sheep adrenal (click the image to flip between surface and cut views): * An inner medulla, which is a source of the catecholamines epinephrine and norepinephrine. The chromaffin cell is the principle cell type. The medulla is richly innervated by preganglionic sympathetic fibers and is, in essence, an extension of the sympathetic nervous system. * An outer cortex, which secretes several classes of steroid hormones (glucocorticoids and mineralocorticoids, plus a few others). Histologic examination of the cortex reveals three concentric zones of cells that differ in the major steroid hormones they secrete. Despite their organization into a single gland, the medulla and cortex are functionally different endocrine organs, and have different embryological origins. The medulla derives from ectoderm (neural crest), while the cortex develops from mesoderm. The utility, if any, of having them together in one discrete organ is not obvious. In some species, amphibians and certain fish, for example, two separate organs are found. Adrenal Medullary Hormones -----------------------------------------------------------------------Cells in the adrenal medulla synthesize and secrete norepinephrine and epinephrine. The ratio of these two catecholamines differs considerably among species: in humans, cats and chickens, roughly 80, 60 and 30% of the catecholamine output is epinephrine. Following release into blood, these hormones bind adrenergic receptors on target cells, where they induce essentially the same effects as direct sympathetic nervous stimulation. Synthesis and Secretion of Catecholamines Synthesis of catecholamines begins with the amino acid tyrosine, which is taken up by chromaffin cells in the medulla and converted to norepinephrine and epinephrine through the following steps: Norepinephine and epinephrine are stored in electron-dense granules which also contain ATP and several neuropeptides. Secretion of these hormones is stimulated by acetylcholine release from preganglionic sympathetic fibers innervating the medulla. Many types of "stresses" stimulate such secretion, including exercise, hypoglycemia and trauma. Following secretion into blood, the catecholamines bind loosely to and are carried in the circulation by albumin and perhaps other serum proteins. XXXXXXXXXXFOTO 42 Adrenergic Receptors and Mechanism of Action The physiologic effects of epinephrine and norepinephrine are initiated by their binding to adrenergic receptors on the surface of target cells. These receptors are prototypical examples of seven-pass transmembrane proteins that are coupled to G proteins which stimulate or inhibit intracellular signalling pathways. Complex physiologic responses result from adrenal medullary stimulation because there are multiple receptor types which are differentially expressed in different tissues and cells. The alpha and beta adrenergic receptors and their subtypes were originally defined by differential binding of various agonists and antagnonists and, more recently, by analysis of molecular clones. Receptor Effectively Binds Effect of Ligand Binding Alpha1 Epinephrine, Norepinphrine Increased free calcium Alpha2 Epinephrine, Norepinphrine Decreased cyclic AMP Beta1 Epinephrine, Norepinphrine Increased cyclic AMP Beta2 Epinephrine Increased cyclic AMP XXXXXXXXXXFFOTOTO 43 Physiologic Effects of Medullary Hormones In general, circulating epinephrine and norepinephrine released from the adrenal medulla have the same effects on target organs as direct stimulation by sympathetic nerves, although their effect is longer lasting. Additionally, of course, circulating hormones can cause effects in cells and tissues that are not directly innervated. The physiologic consequences of medullary catecholamine release are justifiably framed as responses which aid in dealing with stress. These effects can be predicted to some degree by imagining what would be needed if, for example, you were trapped in Jurassic Park when the power went off. A listing of some major effects mediated by epinephrine and norepinephrine are: * Increased rate and force of contraction of the heart muscle: this is predominantly an effect of epinephrine acting through beta receptors. * Constriction of blood vessels: norepinephrine, in particular, causes widespread vasoconstriction, resulting in increased resistance and hence arterial blood pressure. * Dilation of bronchioles: assists in pulmonary ventilation. * Stimulation of lipolysis in fat cells: this provides fatty acids for energy production in many tissues and aids in conservation of dwindling reserves of blood glucose. * Increased metabolic rate: oxygen consumption and heat production increase throughout the body in response to epinephrine. Medullary hormones also promote breakdown of glycogen in skeletal muscle to provide glucose for energy production. * Dilation of the pupils: particularly important in situations where you are surrounded by velociraptors under conditions of low ambient light. * Inhibition of certain "non-essential" processes: an example is inhibition of gastrointestinal secretion and motor activity. Common stimuli for secretion of adrenomedullary hormones include exercise, hypoglycemia, hemorrhage and emotional distress. Adrenal Steroids -----------------------------------------------------------------------The adrenal cortex is a factory for steroid hormones. In total, at least two to three dozen different steroids are synthesized and secreted from this tissue, but two classes are of particular importance: XXXXXXXFOTO 44 Class of Steroid Major Representative Physiologic Effects Mineralocorticoids Aldosterone Na+, K+ and water homeostasis Glucocorticoids Cortisol Glucose homeostasis and many others Additionally, the adrenal cortex produces some sex steroids, particularly androgens, a talent of considerable importance in such diseases as congenital adrenal hyperplasia. Like all steroids, adrenal "corticosteroids" are synthesized from cholesterol through a series of enzyme-mediated transformations. The details of these pathways are presented elsewhere, but the major branches are easy to understand. Each of the three major pathways involves sequential processing by a group of enzymes, some of which reside in endoplasmic reticulum and others inside mitochondria. Hence, synthesis involves shuttling of the steroids between these two organelles. Synthesis of the different steroids is not uniformly distributed through the cortex. For example, the outermost group of cells (zona glomerulosa) synthesizes aldosterone, but essentially no cortisol or androgens because those cells do not express the enzyme 17-alpha-hydroxylase which is necessary for synthesis of 17-hydroxypregnenolone and 17-hydroxyprogesterone. That enzyme is however present in cells of the inner zones of the cortex (zonae fasiculata and reticularis), which are the major sites of cortisol production. FOTO XXXXXXXXXX 45 Like all steroid hormones, cortisol and aldosterone bind to their respective receptors, and the resulting hormone-receptor complexes bind to a hormone response element to modulate transcription of responsive genes. Although the physiologic effects of these two steroid hormones are distinctly different, their receptors are quite similar and, most interestingly, they bind to the same consensus response element in DNA! How then is it possible to get hormone-specific responses? Follow the path to the next topic to find out at least part of the answer. Mineralocorticoids -----------------------------------------------------------------------Removal of the adrenal glands leads to death within just a few days. Observation of such a unfortunate subject would reveal several key derangements: * the concentration of potassium in extracelluar fluid becomes dramatically elevated * urinary excretin of sodium is high and concentrations of sodium in extracellular fluid decreases significantly * volume of extracellular fluid and blood plummet * the heart begins to function poorly, cardiac output declines and shock ensues These phenomena are a direct result of loss of mineralocorticoid activity, and can largely be prevented by replacement of salts and mineralocorticoids. Clearly mineralocorticoids are acutely critical for maintenance of life! Aldosterone and Mineralocorticoid Receptors The principal steroid with mineralocorticoid activity is aldosterone. Cortisol, the major glucocorticoid in non-rodent species, is said to have "weak mineralocorticoid activity", which is of some importance because cortisol is secreted very much more abundantly than aldosterone. Another way to state this is that a small fraction of the mineralocorticoid response in the body is due to cortisol rather than aldosterone. The mineralocorticoid receptor binds both aldosterone and cortisol with equal affinity. Moreover, the same DNA sequence serves as a hormone response element for the activated (steroid-bound) forms of both mineralocorticoid and glucocorticoid receptors. An obvious question is: How in the world can aldosterone stimulate specific biological effects in this kind of system, particularly when blood concentrations of cortisol are something like 2000-fold higher than aldosterone? A large part of the answer is that, in aldosterone-responsive cells, cortisol is effectively destroyed, allowing aldosterone to bind its receptor without competition. Target cells for aldosterone express the enzyme 11-beta-hydroxysteroid dehydrogenase, which has no effect on aldosterone, but converts cortisol to cortisone, which has only a very weak affinity for the mineralocorticoid receptor. In essence, this enzyme "protects" the cell from cortisol and allows aldosterone to act appropriately. Some tissues (e.g. hippocampus) express abundant mineralocorticoid receptors but not 11-beta HSD - they therefore do not show responses to aldosterone because aldosterone is not present in quantities sufficient to compete with cortisol. An interesting demonstration of this enzyme protection system is seen in chronic licorice intoxication. XXXXXXXFOTO 46 Physiologic Effects of Mineralocorticoids Mineralocorticoids play a critical role in regulating concentrations of minerals - particularly sodium and potassium - in extracellular fluids. As described above, loss of these hormones leads rapidly to life-threatening abnormalities in electrolyte and fluid balance. The major target of aldosterone is the distal tubule of the kidney, where it stimulates exchange of sodium and potassium. Three primary physiologic effects result: * Increased resorption of sodium: sodium loss in urine is decreased under aldosterone stimulation. * Increased resorption of water, with consequent expansion of extracellular fluid volume. This is an osmotic effect directly related to increased resorption of sodium. * Increased renal excretion of potassium. Knowing these effects should quickly suggest the cellular mechanism of action this hormone. Aldosterone stimulates transcription of the gene encoding the sodium-potassium ATPase, leading to increased numbers of "sodium pumps" in the basolateral membranes of tubular epithelial cells. Aldosterone also stimulates expression of a sodium channel which facilitates uptake of sodium from the tubular lumen. Aldosterone has effects on sweat glands, salivary glands and the colon which are essentially identical to those seen in the distal tubule of the kidney. The major net effect is again to conserve body sodium by stimulating its resorption or, in the case of the colon, absorption from the intestinal lumen. Conservation of water follows conservation of sodium. Control of Aldosterone Secretion Control over aldosterone secretion is truly multifactorial and tied into a spider web of other factors which regulate fluid and electrolyte composition and blood pressure. If the major effects of aldosterone are considered, it is rather easy to predict factors which stimulate or suppress aldosterone secretion. The two most significant regulators of aldosterone secretion are: * Concentrations of potassium ion in extracellular fluid: Small increases in blood levels of potassium strongly stimulate aldosterone secretion. * Angiotensin II: Activation of the renin-angiotensin system as a result of decreased renal blood flow (usually due to decreased vascular volume) results in release of angiotensin II, which stimulates aldosterone secretion. Other factors which stimulate aldosterone secretion include adrenocorticotropic hormone (short-term stimulation only) and sodium deficiency. Factors which suppress aldosterone secretion include atrial naturetic hormone, high sodium concentration and potassium deficiency. Disease States A deficiency in aldosterone can occur by itself or, more commonly, in conjunction with a glucocorticoid deficiency, and is known as hypoadrenocorticism or Addison's disease. Without treatment by mineralocorticoid replacement therapy, aldosterone deficiency is lethal, due to electrolyte imbalances and resulting hypotension and cardiac failure. Glucocorticoids -----------------------In contrast to loss of mineralocorticoids, failure to produce glucocorticoids is not acutely lifethreatening. Nevertheless, loss or profound diminishment of glucocorticoid secretion leads to a state of deranged metabolism and an inability to deal with stressors which, if untreated, is fatal. In addition to their physiologic importance, glucocorticoids are also among the most frequently used drugs, and often prescribed for their anti-inflammatory and immunosuppressive properties. Cortisol and Glucocorticoid Receptors The vast majority of glucocorticoid activity in most mammals is from cortisol, also known as hydrocortisone. Corticosterone, the major glucocorticoid in rodents, is another glucocorticoid. Cortisol binds to the glucocorticoid receptor in the cytoplasm and the hormone-receptor complex is then translocated into the nucleus, where it binds to its DNA response element and modulates transcription from a battery of genes, leading to changes in the cell's phenotype. Only about 10% of circulating cortisol is free. The remaining majority circulates bound to plasma proteins, particularly corticosteroid-binding globulin (transcortin). This protein binding likely decreases the metabolic clearance rate of glucocorticoids and, because the bound steroid is not biologically active, tends to act as a buffer and blunt wild fluctuations in cortisol concentration. Physiologic Effects of Glucocorticoids There seem to be no cells that lack glucocorticoid receptors and as a consequence, these steroid hormones have a huge number of effects on physiologic systems. That having been said, it can be stated that the best known and studied effects of glucocorticoids are on carbohydrate metabolism and immune function. Effects on Metabolism The name glucocorticoid derives from early observations that these hormones were involved in glucose metabolism. In the fasted state, cortisol stimulates several processes that collectively serve to increase and maintain normal concentrations of glucose in blood. These effects include: * Stimulation of gluconeogenesis, particularly in the liver: This pathway results in the synthesis of glucose from non-hexose substrates such as amino acids and lipids and is particularly important in carnivores and certain herbivores. Enhancing the expression of enzymes involved in gluconeogenesis is probably the best known metabolic function of glucocorticoids. * Mobilization of amino acids from extrahepatic tissues: These serve as substrates for gluconeogenesis. * Inhibition of glucose uptake in muscle and adipose tissue: A mechanism to conserve glucose. * Stimulation of fat breakdown in adipose tissue: The fatty acids released by lipolysis are used for production of energy in tissues like muscle, and the released glycerol provide another substrate for gluconeogenesis. Effects on Inflammation and Immune Function Glucocorticoids have potent anti-inflammatory and immunosuppressive properties. This is particularly evident when they are administered at pharmacologic doses, but also is important in normal immune responses. As a consequence, glucocorticoids are widely used as drugs to treat inflammatory conditions such as arthritis or dermatitis, and as adjunction therapy for conditions such as autoimmune diseases. Other Effects of Glucocorticoids Glucocorticoids have multiple effects on fetal development. An important example is their role in promoting maturation of the lung and production of the surfactant necessary for extrauterine lung function. Mice with homozygous disruptions in the corticotropin-releasing hormone gene (see below) die at birth due to pulmonary immaturity. Excessive glucocorticoid levels resulting from administration as a drug or hyperadrenocorticism have effects on many systems. Some examples include inhibition of bone formation, suppression of calcium absorption and delayed wound healing. These observations suggest a multitide of less dramatic physiologic roles for glucocorticoids. Control of Cortisol Secretion XXXXXXXXXXXXXFOTO 47 Cortisol and other glucocorticoids are secreted in response to a single stimulator: adrenocorticotropic hormone (ACTH) from the anterior pituitary. ACTH is itself secreted under control of the hypothalamic peptide corticotropic-releasing hormone (CRH). The central nervous system is thus the commander and chief of glucocorticoid responses, providing an excellent example of close integration between the nervous and endocrine systems. Virtually any type of physical or mental stress results in elevation of cortisol concentrations in blood due to enhanced secretion of CRH in the hypothalamus. This fact sometimes makes it very difficult to assess glucocorticoid levels, particularly in animals. Observing the approach of a phlebotomist, and especially being restrained for blood sampling, is enough stress to artificially elevate cortisol levels several fold! Cortisol secretion is suppressed by classical negative feedback loops. When blood concentrations rise above a certain theshold, cortisol inhibits CRH secretion from the hypothalamus, which turns off ACTH secretion, which leads to a turning off of cortisol secretion from the adrenal. The combination of positive and negative control on CRH secretion results in pulsatile secretion of cortisol. Typically, pulse amplitude and frequency are highest in the morning and lowest at night. ACTH, also known as corticotropin, binds to receptors in the plasma membrane of cells in the zona fasiculata and reticularis of the adrenal. Hormone-receptor engagement activates adenyl cyclase, leading to elevated intracellular levels of cyclic AMP which leads ultimately to activation of the enzyme systems involved in biosynthesis of cortisol from cholesterol. Disease States The most prevalent disorder involving glucocorticoids in man and animals is Cushings disease or hyperadrenocorticism. Excessive levels of glucocorticoids are seen in two situations: * Excessive endogenous production of cortisol, which can result from a primary adrenal defect (ACTH-independent) or from excessive secretion of ACTH (ACTH-dependent). * Administration of glucocorticoids for theraputic purposes. This is a common side-effect of these widely-used drugs. Cushing's disease has widespread effects on metabolism and organ function, which is not surprising considering the ubiquitious distribution of glucocorticoid receptors. A diverse set of clinicial manifestations accompany this disorder, including hypertension, apparent obesity, muscle wasting, thin skin, and metabolic derangements such as diabetes. Insufficient production of cortisol, often accompanied by an aldosterone deficiency, is called Addison's disease or hypoadrenocorticism. Most commonly, this diease is a result of infectious disease (e.g. tuberculosis in humans) or autoimmune destruction of the adrenal cortex. As with Cushing's disease, numerous diverse clincial signs accompany Addison's disease, including cardiovascular disease, lethargy, diarrhea, and weakness. Aldosterone deficiency can be acutely life threatening due to disorders of electrolyte balance and cardiac function. The Endocrine Pancreas: Introduction and Index -----------------------------------------------------------------------The pancreas houses two distinctly different tissues. The bulk of its mass is exocrine tissue and associated ducts, which produce an alkaline fluid loaded with digestive enzymes which is delivered to the small intestine to facilitate digestion of foodstuffs. Scattered throughout the exocrine tissue are several hundred thousand clusters of endocrine cells which produce the hormones insulin and glucagon, plus a few other hormones. Insulin and glucagon are critical participants in glucose homeostasis and serve as acute regulators of blood glucose concentration. From a medical perspective, insulin in particular is enormously important - a deficiency in insulin or deficits in insulin responsiveness lead to the disease diabetes mellitus. -----------------------------------------------------------------------Functional Anatomy of the Endocrine Pancreas -----------------------------------------------------------------------The pancreas is an elongated organ nestled next to the first part of the small intestine. Its gross anatomy and the structure of pancreatic exocrine tissue and ducts are discussed in the context of the digestive system. The endocrine pancreas refers to those cells within the pancreas that synthesize and secrete hormones. The endocrine portion of the pancreas takes the form of many small clusters of cells called islets of Langerhans or, more simply, islets. Humans have roughly one million islets. In standard histological sections of the pancreas, islets are seen as relatively pale-staining groups of cells embedded in a sea of darker-staining exocrine tissue. The image to the right shows three islets in the pancreas of a horse. Pancreatic islets house three major cell types, each of which produces a different endocrine product: * Alpha cells (A cells) secrete the hormone glucagon. * Beta cells (B cells) produce insulin and are the most abundant of the islet cells. * Delta cells (D cells) secrete the hormone somatostatin, which is also produced by a number of other endocrine cells in the body. Interestingly, the different cell types within an islet are not randomly distributed - beta cells occupy the central portion of the islet and are surrounded by a "rind" of alpha and delta cells. Aside from the insulin, glucagon and somatostatin, a number of other "minor" hormones have been identified as products of pancreatic islets cells. Islets are richly vascularized, allowing their secreted hormones ready access to the circulation. Although islets comprise only 1-2% of the mass of the pancreas, they receive about 10 to 15% of the pancreatic blood flow. Additionally, they are innervated by parasympathetic and sympathetic neurons, and nervous signals clearly modulate secretion of insulin and glucagon Insulin Synthesis and Secretion -----------------------------------------------------------------------Structure of Insulin XXXXXXXXXXXXXFOTO 48 Insulin is a rather small protein, with a molecular weight of about 6000 Daltons. It is composed of two chains held together by disulfide bonds. The figure to the right shows a molecular model of bovine insulin, with the A chain colored blue and the larger B chain green. You can get a better appreciation for the structure of insulin by manipulating such a model yourself. The amino acid sequence is highly conserved amoung vertebrates, and insulin from one mammal almost certainly is biologically active in another. Even today, many diabetic patients are treated with insulin extracted from pig pancreases. Biosynthesis of Insulin Insulin is synthesized in significant quantities only in B cells in the pancreas. The insulin mRNA is translated as a single chain precursor called preproinsulin, and removal of its signal peptide during insertion into the endoplasmic reticulum generates proinsulin. XXXXXXXXXXXFOTO 49 Proinsulin consists of three domains: an amino-terminal B chain, a carboxy-terminal A chain and a connecting peptide in the middle known as the C peptide. Within the endoplasmic reticulum, proinsulin is exposed to several specific endopeptidases which excise the C peptide, thereby generating the mature form of insulin. Insulin and free C peptide are packaged in the Golgi into secretory granules which accumulate in the cytoplasm. When the B cell is appropriately stimulated, insulin is secreted from the cell by exocytosis and diffuses into islet capillary blood. C peptide is also secreted into blood, but has no known biological activity. Control of Insulin Secretion Insulin is secreted in primarily in response to elevated blood concentrations of glucose. This makes sense because insulin is "in charge" of facilitating glucose entry into cells. Some neural stimuli (e.g. site and taste of food) and increased blood concentrations of other fuel molecules, including amino acids and fatty acids, also promote insulin secretion. Our understanding of the mechanisms behind insulin secretion remain somewhat fragmentary. Nonetheless, certain features of this process have been clearly and repeatedly demonstrated, yielding the following model: * Glucose is transported into the B cell by facilitated diffusion through a glucose transporter; elevated concentrations of glucose in extracellular fluid lead to elevated concentrations of glucose within the B cell. * Elevated concentrations of glucose within the B cell ultimately leads to membrane depolarization and an influx of extracellular calcium. The resulting increase in intracellular calcium is thought to be one of the primary triggers for exocytosis of insulin-containing secretory granules. The mechanisms by which elevated glucose levels within the B cell cause depolarization is not clearly established, but seems to result from metabolism of glucose and other fuel molecules within the cell, perhaps sensed as an alteration of ATP:ADP ratio and transduced into alterations in membrane conductance. * Increased levels of glucose within B cells also appears to activate calcium-independent pathways that participate in insulin secretion. Stimulation of insulin release is readily observed in whole animals or people. The normal fasting blood glucose concentration in humans and most mammals is 80 to 90 mg per 100 ml, associated with very low levels of insulin secretion. The figure to the right depicts the effects on insulin secretion when enough glucose is infused to maintain blood levels two to three times the fasting level for an hour. Almost immediately after the infusion begins, plasma insulin levels increase dramatically. This initial increase is due to secretion of preformed insulin, which is soon significantly depleted. The secondary rise in insulin reflects the considerable amount of newly synthesized insulin that is released immediately. Clearly, elevated glucose not only simulates insulin secretion, but also transcription of the insulin gene and translation of its mRNA. XXXXXXXXXXXXXXFOTO 50 Physiologic Effects of Insulin -----------------------------------------------------------------------Stand on a streetcorner and ask people if they know what insulin is, and many will reply, "Doesn't it have something to do with blood sugar?" Indeed, that is correct, but such a response is a bit like saying "Mozart? Wasn't he some kind of a musician?" Insulin is a key player in the control of intermediary metabolism. It has profound effects on both carbohydrate and lipid metabolism, and significant influences on protein and mineral metabolism. Consequently, derangements in insulin signalling have widespread and devastating effects on many organs and tissues. The Insulin Receptor and Mechanism of Action Like the receptors for other protein hormones, the receptor for insulin is embedded in the plasma membrane. The insulin receptor is composed of two alpha subunits and two beta subunits linked by disulfide bonds. The alpha chains are entirely extracellular and house insulin binding domains, while the linked beta chains penetrate through the plasma membrane. XXXXXXXXXXXXXFOTO 51 The insulin receptor is a tyrosine kinase. In other words, it functions as an enzyme that transfers phosphate groups from ATP to tyrosine residues on intracellular target proteins. Binding of insulin to the alpha subunits causes the beta subunits to phosphorylate themselves (autophosphorylation), thus activating the catalytic activity of the receptor. The activated receptor then phosphorylates a number of intracellular proteins, which in turn alters their activity, thereby generating a biological response. Several intracellular proteins have been identified as phosphorylation substrates for the insulin receptor, the best-studied of which is insulin receptor substrate 1 or IRS-1. When IRS-1 is activated by phosphorylation, a lot of things happen. Among other things, IRS-1 serves as a type of docking center for recruitment and activation of other enzymes that ultimately mediate insulin's effects. A more detailed look at these processes is presented in the section on Insulin Signal Transduction. Insulin and Carbohydrate Metabolism Glucose is liberated from dietary carbohydrate such as starch or sucrose by hydrolysis within the small intestine, and is then absorbed into the blood. Elevated concentrations of glucose in blood stimulate release of insulin, and insulin acts on cells thoughout the body to stimulate uptake, utilization and storage of glucose. The effects of insulin on glucose metabolism vary depending on the target tissue. Two important effects are: * Insulin facilitates entry of glucose into muscle, adipose and several other tissues. The only mechanism by which cells can take up glucose is by facilitated diffusion through a family of hexose transporters. In many tissues - muscle being a prime example - the major transporter used for uptake of glucose (called GLUT4) is made available in the plasma membrane through the action of insulin. In the absense of insulin, GLUT4 glucose transporters are present in cytoplasmic vesicles, where they useless for transporting glucose. Binding of insulin to receptors on such cells leads rapidly to fusion of those vesicles with the plasma membrane and insertion of the glucose transporters, thereby giving the cell an ability to efficiently take up glucose. When blood levels of insulin decrease and insulin receptors are no longer occupied, the glucose transporters are recycled back into the cytoplasm. The animation to the right depicts how insulin signalling leads to translocation of glucose transporters from the cytoplasm into the plasma membrane, allowing glucose (small blue balls) to enter the cell. Click on the "Add Glucose" button to start it. It should be noted here that there are some tissues that do not require insulin for efficient uptake of glucose: important examples are brain and the liver. This is because these cells don't use GLUT4 for importing glucose, but rather, another transporter that is not insulindependent. Insulin stimulates the liver to store glucose in the form of glycogen. A large fraction of glucose absorbed from the small intestine is immediately taken up by hepatocytes, which convert it into the storage polymer glycogen. XXXXXXXXFOTO 52 Insulin has several effects in liver which stimulate glycogen synthesis. First, it activates the enzyme hexokinase, which phosphorylates glucose, trapping it within the cell. Coincidently, insulin acts to inhibit the activity of glucose-6-phosphatase. Insulin also activates several of the enzymes that are directly involved in glycogen synthesis, including phosphofructokinase and glycogen synthase. The net effect is clear: when the supply of glucose is abundant, insulin "tells" the liver to bank as much of it as possible for use later. A well-known effect of insulin is to decrease the concentration of glucose in blood, which should make sense considering the mechanisms described above. Another important consideration is that, as blood glucose concentrations fall, insulin secretion ceases. In the absense of insulin, a bulk of the cells in the body become unable to take up glucose, and begin a switch to using alternative fuels like fatty acids for energy. Neurons, however, require a constant supply of glucose, which in the short term, is provided from glycogen reserves. In the absense of insulin, glycogen synthesis in the liver ceases and enzymes responsible for breakdown of glycogen become active. Glycogen breakdown is stimulated not only by the absense of insulin but by the presence of glucagon, which is secreted when blood glucose levels fall below the normal range. Insulin and Lipid Metabolism The metabolic pathways for utilization of fats and carbohydrates are deeply and intricately intertwined. Considering insulin's profound effects on carbohydrate metabolism, it stands to reason that insulin also has important effects on lipid metabolism. Notable effects of insulin on lipid metabolism include the following: * Insulin promotes synthesis of fatty acids in the liver. As discussed above, insulin is stimulatory to synthesis of glycogen in the liver. However, as glycogen accumulates to high levels (roughly 5% of liver mass), further synthesis is strongly suppressed. XXXXXXXXXXFOTO 53 When the liver is saturated with glycogen, any additional glucose taken up by hepatocytes is shunted into pathways leading to synthesis of fatty acids, which are exported from the liver as lipoproteins. The lipoproteins are ripped apart in the circulation, providing free fatty acids for use in other tissues, including adipocytes, which use them to synthesize triglyceride. * Insulin inhibits breakdown of fat in adipose tissue by inhibiting the intracellular lipase that hydrolyzes triglycerides to release fatty acids. Insulin facilitates entry of glucose into adipocytes, and within those cells, glucose can be used to synthesize glycerol. This glycerol, along with the fatty acids delivered from the liver, are used to synthesize triglyceride within the adipocyte. By these mechanisms, insulin is involved in further accumulation of triglyceride in fat cells. From a whole body perspective, insulin has a fat-sparing effect. Not only does it drive most cells to preferentially oxidize carbohydrates instead of fatty acids for energy, insulin indirectly stimulates accumulation of fat is adipose tissue. Other Notable Effects of Insulin In addition to insulin's effect on entry of glucose into cells, it also stimulates the uptake of amino acids, again contributing to its overall anabolic effect. When insulin levels are low, as in the fasting state, the balance is pushed toward intracellular protein degradation. Insulin also increases the permiability of many cells to potassium, magnesium and phosphate ions. The effect on potassium is clinically important. Insulin activates sodium-potassium ATPases in many cells, causing a flux of potassium into cells. Under certain circumstances, injection of insulin can kill patients because of its ability to acutely suppress plasma potassium concentrations. Insulin Deficiency and Excess Diseases Diabetes mellitus, arguably the most important metabolic disease of man, is an insulin deficiency state. It also is a significant cause of disease in dogs and cats. Two principal forms of this disease are recognized: * Type I or insulin-dependent diabetes mellitus is the result of a frank deficiency of insulin. The onset of this disease typically is in childhood. It is due to destruction pancreatic B cells, most likely the result of autoimmunity to one or more components of those cells. Many of the acute effects of this disease can be controlled by insulin replacement therapy, but inevitably, there are long-term adverse effects on blood vessels, nerves and other organ systems. * Type II or non-insulin-dependent diabetes mellitus begins as a syndrome of insulin resistance. That is, target tissues fail to respond appropriately to insulin. Typically, the onset of this disease is in adulthood. Despite monumental research efforts, the nature of the defect has been difficult to ascertain - in some patients, the insulin receptor is abnormal, in others, one or more aspects of insulin signalling is defective, and in others, no defect has been identified. Because there is not, at least initially, an inability to secrete adequate amounts of insulin, insulin injections are not useful for therapy. Rather the disease is controlled through dietary therapy and hypoglycemic agents. Hyperinsulinemia or excessive insulin secretion is usually the result of an insulin-secreting tumor. This condition is much less common than diabetes mellitus. The high levels of insulin resulting from this condition or from an overdose of insulin causes a precipitious drop in blood glucose concentrations. The brain becomes starved for energy, leading to the syndrome of insulin shock, which is acutely life-threatening. -----------------------------------------------------------------------Glucagon -----------------------------------------------------------------------Glucagon has a major role in maintaining normal concentrations of glucose in blood, and is often described as having the opposite effect of insulin. That is, glucagon has the effect of increasing blood glucose levels. XXXXXXXXXXXXXFOTO 54 Glucagon is a linear peptide of 29 amino acids. Its primary sequence is almost perfectly conserved among vertebrates, and it is structurally related to the secretin family of peptide hormones. Glucagon is synthesized as proglucagon and proteolytically processed to yield glucagon within alpha cells of the pancreatic islets. Proglucagon is also expressed within the intestinal tract, where it is processed not into glucagon, but to a family of glucagon-like peptides (enteroglucagon). Physiologic Effects of Glucagon The major effect of glucagon is to stimulate an increase in blood concentration of glucose. As discussed previously, the brain in particular has an absolute dependence on glucose as a fuel, because neurons cannot utilize alternative energy sources like fatty acids to any significant extent. When blood levels of glucose begin to fall below the normal range, it is imperative to find and pump additional glucose into blood. Glucagon exerts control over two pivotal metabolic pathways within the liver, leading that organ to dispense glucose to the rest of the body: XXXXXXXXXXXXXXXXFOTO 55 * Glucagon stimulates breakdown of glycogen stored in the liver. When blood glucose levels are high, large amounts of glucose are taken up by the liver. Under the influence of insulin, much of this glucose is stored in the form of glycogen. Later, when blood glucose levels begin to fall, glucagon is secreted and acts on hepatocytes to activate the enzymes that depolymerize glycogen and release glucose. * Glucagon activates hepatic gluconeogenesis. Gluconeogenesis is the pathway by which non-hexose substrates such as amino acids are converted to glucose. As such, it provides another source of glucose for blood. This is especially important in animals like cats and sheep that don't absorb much if any glucose from the intestine - in these species, activation of gluconeogenic enzymes is the chief mechanism by which glucagon does its job. Glucagon also appears to have a minor effect of enhancing lipolysis of triglyceride in adipose tissue, which could be viewed as an addition means of conserving blood glucose by providing fatty acid fuel to most cells. Control of Glucagon Secretion Knowing that glucagon's major effect is to increase blood glucose levels, it makes sense that glucagon is secreted in response to hypoglycemia or low blood concentrations of glucose. Two other conditions are known to trigger glucagon secretion: * Elevated blood levels of amino acids, as would be seen after consumption of a protein-rich meal: In this situation, glucagon would foster conversion of excess amino acids to glucose by enhancing gluconeogenesis. Since high blood levels of amino acids also stimulate insulin release, this would be a situation in which both insulin and glucagon are active. * Exercise: In this case, it is not clear whether the actual stimulus is exercise per se, or the accompanying exercise-induced depletion of glucose. In terms of negative control, glucagon secretion is inhibited by high levels of blood glucose. It is not clear whether this reflects a direct effect of glucose on the alpha cell, or perhaps an effect of insulin, which is known to dampen glucagon release. Another hormone well known to inhibit glucagon secretion is somatostatin. Disease States Diseases associated with excessively high or low secretion of glucagon are rare. Cancers of alpha cells (glucagonomas) are one situation known to cause excessive glucagon secretion. These tumors typically lead to a wasting syndrome and, interestingly, rash and other skin lesions. Although insulin deficiency is clearly the major defect in type 1 diabetes mellitus, there is considerable evidence that aberrant secretion of glucagon contributes to the metabolic derangements seen in this important disease. For example, many diabetic patients with hyperglycemia also have elevated blood concentrations of glucagon, but glucagon secretion is normally suppressed by elevated levels of blood glucose. Gastrointestinal Hormones: -------------------------------------Digesting, absorbing and assimilating a meal requires precise coordination of a huge number of physiologic processes. Control over gastrointestinal function is, as one would expect, provided by nervous and endocrine systems. The hormones most important in controlling digestive function are synthesized within the gastrointestinal tract by cells scattered in the epithelium of the stomach and small intestine. These endocrine cells and the hormones they secrete are referred to as the enteric endocrine system. Interestingly, most if not all "GI hormones" are also synthesized in the brain. Overview of Gastrointestinal Hormones -----------------------------------------------------------------------If you are like most people, you eat several meals and occasional snacks each day, but rarely think about the immense number of tasks that must be performed by your digestive system to break down, absorb and assimilate those nutrients. Robust control systems are required to coordinate digestive processes in man and animals, and are provided by both the nervous and endocrine systems. Endocrine control over digestive functions is provided by the so-called enteric endocrine system, which is summarized elsewhere. There are a bunch of hormones, neuropeptides and neurotransmitters that affect gastrointestinal function. Interestingly, a number of the classical GI hormones are also synthesized in the brain, and sometimes referred to as "brain-gut peptides". The significance of this pattern of expression is not clear. The following table summarizes the effects and stimuli for release of the major gastrointestinal hormones, each of which is discussed in more detail on subsequent pages: Hormone Major Activities Stimuli for Release Gastrin Stimulates gastric acid secretion and proliferation of gastric epithelium Presence of peptides and amino acids in gastric lumen Cholecystokinin Stimulates secretion of pancreatic enzymes, and contraction and emptying of the gall bladder Presence of fatty acids and amino acids in the small intestine Secretin Stimulates secretion of water and bicarbonate from the pancreas and bile ducts Acidic pH in the lumen of the small intestine Motilin Apparently involved in stimulating housekeeping patterns of motility in the stomach and small intestine Not clear, but secretion is associated with fasting Gastric inhibitory polypeptide Inhibits gastric secretion and motility and potentiates release of insulin from beta cells in response to elevated blood glucose concentration Presence of fat and glucose in the small intestine XXXXXXXXXXXXFOTO 56 Gastrin -----------------------------------------------------------------------Gastrin is a major physiological regulator of gastric acid secretion. It also has an important trophic or growth-promoting influence on the gastric mucosa. Gastrin is synthesized in G cells, which are located in gastric pits, primarily in the antrum region of the stomach and binds receptors found predominantly on parietal and enterochromaffin-like cells. Structure of Gastrin and the Gastrin Receptor Gastrin is a linear peptide that is synthesized as a preprohormone and is posttranslationally cleaved to form a family of peptides with identical carboxytermini. The predominant circulating form is gastrin-34 ("big gastrin"), but full biologic activity is present in the smallest peptide (gastrin-14 or minigastrin). Further, full bioactivity is preserved in the five C-terminal amino acids of gastrin, which is known as pentagastrin. Importantly, the five Cterminal amino acids of gastrin and cholecystokinin are identical, which explains their overlapping biological effects. The gastrin receptor is also one of the receptors that binds cholecystokinin, and known as the CCK-B receptor. Control and Physiologic Effects of Gastrin The primary stimulus for secretion of gastrin is the presence of certain foodstuffs, especially peptides, certain amino acids and calcium, in the gastric lumen. Also, as yet unidentified compounds in coffee, wine and beer are potent stimulants for gastrin secretion. Secretion of this hormone is inhibited when the lumenal pH of the stomach becomes very low (less than about 3). Gastrin receptors are found on parietal cells, and binding of gastrin, along with histamine and acetylcholine, leads to fully-stimulated acid secretion by those cells. Canine parietal cells have roughly 44,000 gastrin receptors each, and in that species, it has been demonstrated that immunoneutralization of gastrin blocks secretion of acid in response to intragastric administration of peptides. Enterochromaffin-like (ECL) cells also bear gastrin receptors, and recent evidence indicates that this cell may be the most important target of gastrin with regard to regulating acid secretion. Stimulation of ECL cells by gastrin leads to histamine release, and histamine binding to H2 receptors on parietal cells is necessary for full-blown acid secretion. In addition to the parietal and ECL cell targets, gastrin also stimulates pancreatic acinar cells via binding to cholecystokinin receptors, and gastrin receptors have been demonstrated on certain populations of gastric smooth muscle cells, supporting pharmacologic studies that demonstrate a role for gastrin in regulating gastric motility. Promotion of gastric mucosal growth has been demonstrated by administration of gastrin to rats. This treatment stimulated DNA, RNA and protein synthesis in gastric mucosa and increased numbers of parietal cells; this effect was blocked by a gastrin/CCK antagonist. Another observation supporting this function is that humans with hypergastrinemia consistently show gastric mucosal hypertrophy. The mechanism of signal transduction by gastrin has not been well characterized. In canine parietal cells, binding of gastrin stimulates production of inositol phosphate and increases in intracellular Ca++. It also results in activation of protein kinase C. Additional studies on mechanism of gastrin action are clearly needed. Disease States Excessive secretion of gastrin, or hypergastrinemia, is a well-recognized cause of a severe disease known as Zollinger-Ellison syndrome, which is seen at low frequency in man and dogs. The hallmark of this disease is gastric and duodenal ulceration due to excessive and unregulated secretion of gastric acid. Most commonly, hypergastrinemia is the result of gastrin-secreting tumors (gastrinomas), which develop in the pancreas or duodenum. Cholecystokinin ------------------------------------------------------------------------ Cholecystokinin plays a key role in facilitating digestion within the small intestine. It is secreted from mucosal epithelial cells in the first segment of the small intestine (duodenum), and stimulates delivery into the small intestine of digestive enzymes from the pancreas and bile from the gallbladder. Cholecystokinin is also produced by neurons in the enteric nervous system, and is widely and abundantly distributed in the brain. Structure of Cholecystokinin and Its Receptors As mentioned previously, cholecystokinin and gastrin are highly similar peptides. Like gastrin, cholecystokinin is a linear peptide that is synthesized as a preprohormone, then proteolytically cleaved to generate a family of peptides having the same carboxy ends. Full biologic activity is retained in CCK-8 (8 amino acids), but peptides of 33, 38 and 59 amino acids are also produced. In all of these CCK peptides, the tyrosine seven residues from the end is sulfated, which is necessary for activity. Two receptors that bind cholecystokinin have been identified. The CCKA receptor is found abundantly on pancreatic acinar cells. The CCKB receptor, which also functions as the gastrin receptor, is the predominant form in brain and stomach. Both receptors are have seven transmembrane domains typical of G protein-coupled receptors. Control and Physiologic Effects of Cholecystokinin Foodstuffs flowing into the small intestine consist mostly of large macromolecules (proteins, polysaccharides and triglyceride) that must be digested into small molecules (amino acids, monosaccharides, fatty acids) in order to be absorbed. Digestive enzymes from the pancreas and bile salts from the liver (which are stored in the gallbladder) are critical for such digestion. Cholecystokinin is the principle stimulus for delivery of pancreatic enzymes and bile into the small intestine. The most potent stimuli for secretion of cholecystokinin are the presence of partially-digested fats and proteins in the lumen of the duodenum (a particularly potent stimulus is pictured above). An elevation in blood concentration of cholecystokinin has two major effects that facilitate digestion: Release of digestive enzymes from the pancreas into the duodenum. Older literature refers to cholecystokinin as pancreozymin, a term coined to describe this effect. XXXXFOTO 57 * Contraction of the gallbladder to deliver bile into the duodenum. The name cholecystokinin (to "move the gallbladder") was given to describe this effect. Cholecystokinin is also known to stimulate secretion of bile salts into the biliary system. Pancreatic enzymes and bile flow through ducts into the duodenum, leading to digestion and absorption of the very molecules that stimulate cholecystokinin secretion. Thus, when absorption is completed, cholecystokinin secretion ceases. Injection of cholecystokinin into the ventricles of the brain induces satiety (lack of hunger) in laboratory animals. In view of its pattern of secretion relative to feeding, it would make physiologic sense that this hormone might participate in control of food intake. However, recent experiments suggest that cholecystokinin is at best a minor player in regulation of food intake. In addition to its synthesis in small intestinal epithelial cells, cholecystokinin has been clearly demonstrated in neurons within the wall of the intestine and in many areas of the brain. It seems, in fact, to be the most abundant neuropeptide in the central nervous system. Secretion of cholecystokinin from neurons appears to modulate the activity of other hormones and neuropeptides, but it seems safe to say the understanding its role in function of the brain is rudimentary at best. Disease States Diseases resulting from excessive or deficient secretion of cholecystokinin are rare. Cholecystokinin deficiency has been described in humans as part of autoimmune polyglandular syndrome, and was manifest as a malabsorption syndrome clinically similar to pancreatic exocrine insufficiency. Additionally, there is mounting evidence that aberrations in expression of cholesystokinin or its receptor within the human brain may play a part in the pathogenesis of certain types of anxiety and schizophrenia. Clearly, a much better understanding of the role of cholecystokinin in brain function is required. -----------------------------------------------------------------------Secretin -----------------------------------------------------------------------The small intestine is periodically assaulted by a flood of acid from the stomach, and it is important to put out that fire in a hurry to avoid acid burns. Secretin functions as a type of fireman: it is released in response to acid in the small intestine, and stimulates the pancreas to release a flood of bicarbonate base, which neutralizes the acid.. Secretin is also of some historical interest, as it was the first hormone to be discovered. Structure of Secretin and Its Receptors Secretin is synthesized as a preprohormone, then proteolytically processed to yield a single 27-amino acid peptide by removal of the signal peptide plus amino and carboxy-terminal extensions. The sequence of the mature peptide is related to that of glucagon, vasoactive intestinal peptide and gastric inhibitory peptide. The secretin receptor has seven membrane-spanning domains and characteristics typical of a G protein-coupled receptor. Control and Physiologic Effects of Secretin Secretin is secreted in response to one known stimulus: acidification of the duodenum, which occurs most commonly when liquified ingesta from the stomach are released into the small intestine. The principal target for secretin is the pancreas, which responds by secreting a bicarbonate-rich fluid, which flows into the first part of the intestine through the pancreatic duct. Bicarbonate ion is a base and serves to neutralize the acid, thus preventing acid burns and establishing a pH conducive to the action of other digestive enzymes. A similar, but quantitatively less important response to secretin is elicited by bile duct cells, resulting in additional bicarbonate being dumped into the small gut. As acid is neutralized by bicarbonate, the intestinal pH rises toward neutrality, and secretion of secretin is turned off. Disease States Diseases associated with excessive or deficient secretion of secretin are not recognized. Secretin has recently elicited considerable attention by the press due to alleged benefits of a single dose of secretin in treating autism in children. At the time of this writing, only a single, placebo-controlled trial of secretin treatment in autistic children has been reported. That study failed to detect a beneficial effect of secretin, either in the group of autistic children as a whole or in any individual child (Sandler, et al. New Eng J Med 341:1801, 1999). Motilin -----------------------------------------------------------------------Motilin is a 22 amino acid peptide secreted by endocrinocytes in the mucosa of the proximal small intestine. Based on amino acid sequence, motilin is unrelated to other hormones. Motilin participates in controlling the pattern of smooth muscle contractions in the upper gastrointestinal tract. There are two basic states of motility of the stomach and small intestine: the fed state, when foodstuffs are present, and the interdigestive state between meals. Motilin is secreted into the circulation during the fasted state at intervals of roughly 100 minutes. These bursts of motilin secretion are temporily related to the onset of "housekeeping contractions", which sweep the stomach and small intestine clear of undigested material (also called the migrating motor complex). Control of motilin secretion is largely unknown, although some studies suggest that an alkaline pH in the duodenum stimulates its release. An interesting aspect of the motilin story is that erythromycin and related antibiotics act as nonpeptide motilin agonists, and are sometimes used for their ability to stimulate gastrointestinal motility. Administration of a low dose of erythromycin will induce a migrating motor complex, which provides additional support for the conclusion that motilin secretion triggers this pattern of GI motility, rather than results from it.