Chemistry Module 1- Basic Revision Notes
1.1a Atomic Structure
1.1.1 All matter is composed of atoms – very small particles.
The atoms are composed of 3 sub-atomic particles called:(i) Protons – located in the centre of the atom
(ii) Neutrons – located in the centre of the atom
(iii) Electrons – orbiting around the nucleus
The nucleus is the concentration of mass at its centre i.e. protons and neutrons.
The electrons orbit the nucleus
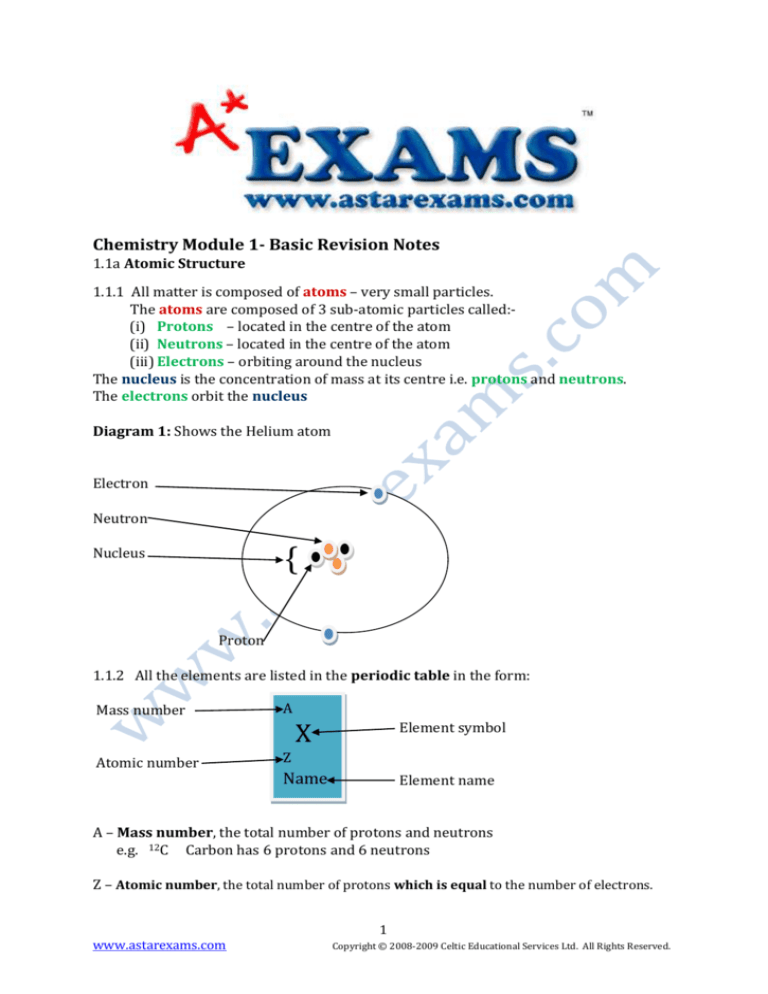
Diagram 1: Shows the Helium atom
Electron
Neutron
{
Nucleus
Proton
1.1.2 All the elements are listed in the periodic table in the form:
Mass number
Atomic number
A
X
Element symbol
Z
Name
Element name
A – Mass number, the total number of protons and neutrons
e.g. 12C Carbon has 6 protons and 6 neutrons
Z – Atomic number, the total number of protons which is equal to the number of electrons.
www.astarexams.com
1
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
e.g.
6C
Carbon has 6 protons = 6 electrons (for a neutral atom)
The electronic structure of atoms can be written in two ways.
1. Electronic notation e.g. 3Li would be 2.1
11Na would be 2.8.1
19K would be 2.8.8.1
NB The first orbit, or shell, can only hold 2 electrons.
The second orbit can hold a maximum of 8 electrons.
The third shell will also hold up to 8 electrons.
2. Electron shell diagrams e.g.
X
X
X
X
X
X
X
lithium(Li): 2.1
X
X
X
X
X
X
X
X
X
Sodium(Na): 2.8.1
X
X
X
X
X
X
X
X
www.astarexams.com
X
X
X
X
X
X
X
Potassium(K): 2.8.8.1
X
X
2
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
1.1b Elements and the Periodic Table
1.1.3 Elements (H, He, Li, Be,…..) are the basic building blocks of all matter, and cannot
be broken down into simpler parts by chemical means.
1.1.4 There is a clear relationship between an elements electronic structure and its
position in the periodic table.
P
E
r
i
o
d
1
2
3
4
The Periodic Table (First 4 rows only)
Group No
I
1H
1
3Li
2.1
11Na
2.8.1
19K
2.8.8.1
II
4Be
III
5B
IV
V
6C
7N
VI
8O
2.2
2.3
2.4
2.5
2.6
12Mg
13Al
14Si
15P
16S
2.8.2
2.8.3
2.8.4
2.8.5
2.8.6
2oCa
2.8.8.2
(The transition metals have been left out)
VII
9F
2.7
17Cl
2.8.7
VIII
2He
2
10Ne
2.8
18Ar
2.8.8
By looking at the above table, you can see that the last number in the electronic
structure, corresponds with the group number e.g. 2.1 = group I
2.5 = group V
Also the electronic structure gives the period number
e.g. 2.1 = period 2
2.8.1 = period 3
Plus the total number of electrons gives the atomic number.
e.g. 2.1 = atomic number 3
2.5 = atomic number 7
Here are some more examples from the periodic table above:Element
3Li
15P
13Al
18Ar
20Ca
www.astarexams.com
Electronic Structure
2.1
2.8.5
2.8.3
2.8.8
2.8.8.2
Group Number
I
V
III
VIII
II
Period Number
2
3
3
3
4
3
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
1.1.5 Mendeléev’s Discoveries
He observed repeating patterns in the properties of elements, when arranged
in order of increasing relative atomic mass.
But he was cleaver and left gaps for elements that had not yet been found.
This allowed him to predict the properties of the unknown elements.
1.1.6 Metals and Non-metals
Properties of Metals
Good conductor of electricity
Good conductor of heat
High melting points
High boiling points
Shiny (Luster)
Some are magnetic
Sonorous (Ringing sound)
Ductile (Drawn into a wire)
Malleable (Easily hammered into shape)
Properties of Non-Metals
Good electrical insulator
Good heat insulator
Low melting points
Low boiling points
Dull
If you know the above properties then telling the difference between metals and nonmetals should be relatively easy. But there are exceptions to look out for:(i) Carbon (graphite)
- good conductor of electricity
(ii) Mercury
-liquid metal (i.e. low melting point)
www.astarexams.com
4
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
1.1.7-8 Properties and Reactions of Group1 – the alkali metals
Group I metal
Lithium
Sodium
Potassium
Rubidium
Caesium
Francium
Atomic
Number
3
11
19
37
55
87
Density Melting Point
(g cm-3)
(oC)
0.53
180
0.97
98
0.89
63
1.50
39
1.93
28
2.40
27
Boiling Point
(oC)
1360
900
777
705
678
677
These metals are the most chemically reactive group of metals and increase in
reactivity down the group, they also,
are known as soft metals(i.e. can be cut easily by a knife)
are low in density(i.e. they even float on water)
are stored under paraffin (oil) due to their high reactivity with water
react quickly with oxygen (in the air)and tarnish
are shiny when newly cut open
Reactions of Group I
(a) Alkali metal with water
General Equation: Metal + Water
e.g.
2Na (s) + 2H2O (l)
Metal Hydroxide + Hydrogen gas
2NaOH (aq) + H2 (g) (‘pop’ test for hydrogen!)
(Solution made has a high pH)
The reaction type and balancing stays the same for all Group I metals i.e.
2Fr (s) + 2H2O (l)
2FrOH (aq) + H2 (g)
(b) Alkali metal with oxygen
General equation: Metal + Oxygen
Metal Oxide
e.g.
4Na (s) + O2 (g)
2Na2O (s) (Also known as Oxidation)
The reaction type and balancing stays the same for all Group I metals i.e.
4Fr (s) + O2 (g)
www.astarexams.com
2Fr2O (s)
5
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
(c) Alkali metal with halogen
General Equation: Metal + Halogen
e.g.
2Na (s) + Cl2 (g)
2NaCl (s)
Salt
The reaction type and balancing stays the same for all Group I metals i.e.
2Fr (s) + Cl2 (g)
2FrCl (s) (Remember that the Halogens are
diatomic! i.e. Cl2, Br2)
The latter equations are very common in GCSE examinations.
Also, you should be prepared to write the word equations.
(d) Diatomic molecules
These molecules are very common in GCSE symbol equations.
Many of them tend to be gases.
The following list of diatomic molecules should be known and learned:-
H2, N2, O2, F2, Cl2, Br2, I2, and At2.
Remember that Bromine is a liquid at room temperature, whilst
Iodine and Astatine are solids at room temperature.
The above diatomic molecules have only one element type.
But there are ones that are not made up of the same elements e.g.
CO – carbon monoxide,
NO – nitrogen oxide.
www.astarexams.com
6
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
Properties and Reactions of Group VII – the halogens
Properties
Halogen
molecule
Fluorine
Chlorine
Bromine
Iodine
Astidine
Formula
F2
Cl2
Br2
I2
At2
Colour
Air
Yellow
Yellow/Green
Red
Black
Black
Water
Yellow/Green
Orange
Brown
Unknown
State
Gas
Gas
Liquid
Solid
Solid
Melting Point
(oC)
–188
–34
58
183
302
The halogens are the most reactive non-metals it’s no wonder that the chemistry with
Group I is so important and extensive.
The Halogens: become less reactive going down the group
the vapours of the halogens are (i) Smelly
(ii) Coloured
(iii) Diatomic
(iv) Poisonous
www.astarexams.com
7
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.
Reactions of Group VII
(a) The halogens with water
chlorine + water
Cl2 (g) + H2O (l)
hydrochloric acid + chloric(I) acid (Bleach)
HCl (aq)
+
HOCl (aq)
(b) Displacement reactions
The word and symbol equations together with the colours produced needs to be
learned well.
Halide Salt Solutions
Halogen Solutions
Chlorine solution
Bromine solution
Iodine solution
Potassium
Chloride
No Reaction
(iii)
(v)
Potassium
Bromide
(i)
No Reaction
(vi)
(i) Cl2 (aq) + 2KBr (aq)
Green
Solution
2KCl (aq) + Br2 (aq)
Orange
Solution
(ii) Cl2 (aq) + 2KI (aq)
Green
Solution
2KCl (aq) + I2 (aq)
Brown
Solution
(iii) Br2 (aq) + KCl (aq)
Orange
Solution
No displacement
Orange
Solution
(iv) Br2 (aq) + KI (aq)
Orange
Solution
2KBr (aq) + I2 (aq)
Brown
Solution
(v)
I2 (aq) + KCl (aq)
Brown
Solution
No displacement
Brown
Solution
(vi) I2 (aq) + KBr (aq)
Brown
Solution
No displacement
Brown
Solution
Potassium
Iodide
(ii)
(iv)
No Reaction
NB The halide salt solutions are all colourless.
www.astarexams.com
8
Copyright © 2008-2009 Celtic Educational Services Ltd. All Rights Reserved.







