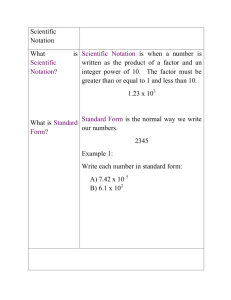
Quiz-CP Review Day 1 The first step of the scientific method is to
advertisement

Quiz-CP Review Day 1 1. The first step of the scientific method is to form the hypothesis. a. True b. False 2. Color is considered a a. Quantitative measurement b. Qualitative measurement 3. A meniscus is a. A lab tool used to transfer chemicals from one container to another b. The pan used to hold the chemicals on a triple beam balance c. A point of reference to accurately measure liquids in a graduated cylinder 4. Absolute zero is a. The point where water freezes b. The point where all motion stops c. The point where water boils d. None of the above 5. The results of a student trying to find the density of lead in lab are below. The actual density of lead is 11.34 g/mL. Which of the below best describes the data? Trial 1 12.48 g/mL a. Low accuracy, low precision Trial 2 12.46 g/mL b. Low accuracy, high precision Trial 3 12.50 g/mL c. High accuracy, low precision d. High accuracy, high precision 6. Calculate the percent error from trial 1 above. a. 10.05% b. 9.13% c. -10.05 % d. -9.13% 7. Convert 34 cm to m a. 0.34 m b. 3.4 m c. 340 m d. 3400 m 8. If a number is in scientific notation with a negative exponent, you know that the number in standard notation will be a. Bigger than one b. Smaller than one c. Need more information 9. Change to scientific notation: 34,000,000 a. 34 x 106 b. 34 x 10-6 c. 3.4 x 107 d. 3.4 x 10-7 e. 3.4 x 106 f. 3.4 x 10-6 10. The number 0.002010 has ____ significant figures. The number 2010 has ____ significant figures. a. 7, 4 b. 7, 3 c. 6, 4 d. 6, 3 e. 3, 4 f. 3, 3 g. 4, 4 h. 4, 3 Quiz-CP Review Day 1 1. The first step of the scientific method is to form the hypothesis. a. True b. False 2. Color is considered a a. Quantitative measurement b. Qualitative measurement 3. A meniscus is a. A lab tool used to transfer chemicals from one container to another b. The pan used to hold the chemicals on a triple beam balance c. A point of reference to accurately measure liquids in a graduated cylinder 4. Absolute zero is a. The point where water freezes b. The point where all motion stops c. The point where water boils d. None of the above 5. The results of a student trying to find the density of lead in lab are below. The actual density of lead is 11.34 g/mL. Which of the below best describes the data? Trial 1 12.48 g/mL a. Low accuracy, low precision Trial 2 12.46 g/mL b. Low accuracy, high precision Trial 3 12.50 g/mL c. High accuracy, low precision d. High accuracy, high precision 6. Calculate the percent error from trial 1 above. a. 10.05% b. 9.13% c. -10.05 % d. -9.13% 7. Convert 34 cm to m a. 0.34 m b. 3.4 m c. 340 m d. 3400 m 8. If a number is in scientific notation with a negative exponent, you know that the number in standard notation will be a. Bigger than one b. Smaller than one c. Need more information 9. Change to scientific notation: 34,000,000 a. 34 x 106 b. 34 x 10-6 c. 3.4 x 107 d. 3.4 x 10-7 e. 3.4 x 106 f. 3.4 x 10-6 10. The number 0.002010 has ____ significant figures. The number 2010 has ____ significant figures. a. 7, 4 b. 7, 3 c. 6, 4 d. 6, 3 e. 3, 4 f. 3, 3 g. 4, 4 h. 4, 3