OrganicChemPaperLab - Mr-Paullers-wiki
advertisement

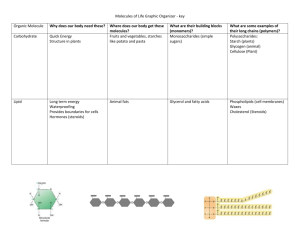
Name___________________________________________________Period__ Chemistry of Organic Molecules bi·o·chem·is·try n. 1. The study of the chemical substances and vital processes occurring in living organisms; biological chemistry; physiological chemistry. 2. The chemical composition of a particular living system or biological substance: viral biochemistry. Objectives: a. apply knowledge of simple molecules (water) to answer questions about organic molecules b. distinguish between paper molecule models and actual chemical formulas of molecules c. determine the molecular characteristics of carbohydrates, fats/lipids, proteins and nucleic acids. Materials: Scissors Procedure: Part A. Water . Chemical formula = H2O Q: What elements make up water? A: H represents hydrogen, O represents oxygen. Q: What does the number 2 following the H mean? A: The two is called a subscript and it tells you that there are two atoms of hydrogen in water Q: Why doesn’t oxygen have a subscript? A: When the symbol for an element is not followed by a subscript, it means the molecule contains one atom of that element. Q: How many molecules of water are represented by H2O? A: One. A coefficient is used if the number of molecules is more than one. For example, 3H2O means that there are 3 molecules of water. Q: What is a structural formula? What is the structural formula of water? A: A structural formula attempts to show the 3-D arrangement of atoms in the molecule. The structural formula of water is. Q: What do the dashes between the O and the H mean? A: The lines represent covalent (shared electrons) chemical bonds. A single line means that one pair of electrons is being shared. = would mean a double bond, ≡ means a triple bond. Q: What does a 3-D representation of water look like? A: Part B. Carbohydrates Carbohydrates contain Carbon (C), Oxygen (O), and Hydrogen (H). Saccharides are also called sugars of carbohydrates. Sugars are placed into one of three categories; monosaccharides, disaccharides, and polysaccharides. Note: mono = 1, di = 2, poly = more than 2. Monosaccharides or Single Sugars: Examine the structural formulas and 3-D representations of sugar molecules to answer the following questions. Note: where lines meet there is a C unless otherwise noted. See board example. Glucose Fructose Structural formula 3-D representation 1. What elements are present in monosaccharides? 2. Write the chemical formulas for each of the three provided examples of monosaccharides. Glucose: C H O Fructose: C H O 3. What is the ratio of hydrogen to oxygen in these molecules? 4. How do the ratios of H to O compare in fructose and glucose? 5. How do they compare to the ratio of H to O in water? 6. What percentage (approximately) of all bonds in these carbohydrates are C-H bonds? _____C-H bonds x 100 = total bonds 7. How are these monosaccharides different? How are they similar? Disaccharides and Polysaccharides Two monosaccharides can combine to form a disaccharide. When more than two monosaccharides are joined, a polysaccharide is formed. Sucrose Tape of paste your model of sucrose here: 1. What is the approximate ratio of C:H:O in a disaccharide? _____________ 2. Cut out a glucose and a fructose paper model. Cut along solid lines only! Can you join the molecules together? _____________ 3. In order to join the molecules, remove and –OH and from one molecule and a –H end from the other. Cut along the dotted lines. Does this help to join the monosaccharides? _________ 4. What compound will be formed with the leftover H and OH? ______________ 5. What portion of the bonds in sucrose are C-H bonds? 6. Construct a portion of a starch molecule by cutting out and joining three glucose molecules. Tape or paste your starch molecule at the top of the next page: 7. What is the formula of starch? C___H___O___ Hint: it’s three glucoses joined, minus 2 waters 8. Why is the process of building disaccharides or polysaccharides called dehydration synthesis? Dehydration means: ___________________________ Synthesis means: ____________________________ Part C. Lipids/Fats Lipids are made mostly of C and H. They store a great deal of energy. 1. What is the difference between saturated and unsaturated fatty acid chains? 2. In a lipid, what percentage of the total number of bonds are C-H bonds? 3. How does this compare to the percentage of bonds in carbohydrates that are C-H bonds? 4. C-H bonds are the most important bond for determining how much energy a molecule can store. The more C-H bonds, the more energy the molecule can store. Rank carbohydrates, saturated fats and unsaturated fats according to how much energy each stores. Stores least amount of energy Stores the most energy ______________________ ______________________ ______________________ Part D. Nucleic Acids. Nucleic acids store and transmit hereditary, or genetic, information. 1. What is RNA short for: _____________________________________________ 2. What is DNA short for: _____________________________________________ 3. How are DNA and RNA similar? 4. How are DNA and RNA different? 5. Label the 3 parts of the nucleotide shown below. Sugar, phosphate, nitrogenous base. Part E. Proteins. Proteins are molecules made of nitrogen (N), as well as C, H, and O. Proteins are polymers of amino acids. 1. List and describe four important jobs performed by proteins. a. _________________________________________________________________ b. _________________________________________________________________ c. _________________________________________________________________ d. _________________________________________________________________ 2. What are the four levels of organization found in proteins? Explain each. a. _________________________________________________________________ b. _________________________________________________________________ c. _________________________________________________________________ d. _________________________________________________________________ 3. Circle and label the R-group, amino group, and carboxyl group of each of the three amino acids shown above. 4. How are cysteine, leucine and valine the same? 5. How are cysteine, leucine and valine different? Formation of a peptide bond: 6. Describe the events that happen during the formation of a peptide bond. 7. Why is a protein sometimes called a polypeptide?