8: Cycling WebQuest
advertisement
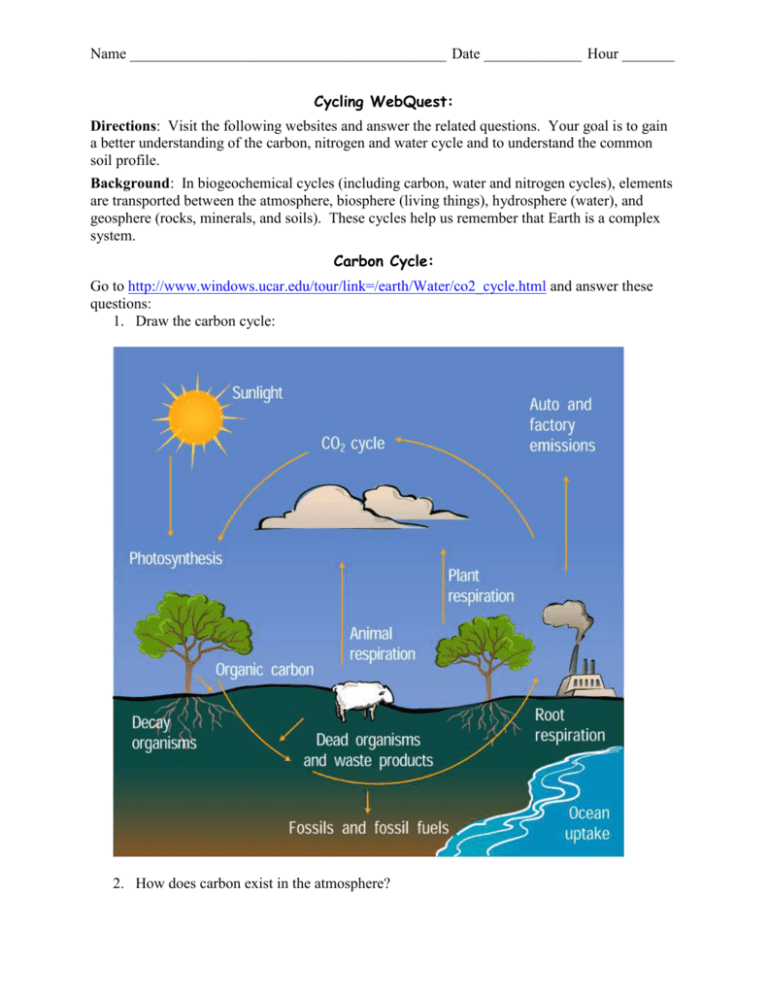
Name __________________________________________ Date _____________ Hour _______ Cycling WebQuest: Directions: Visit the following websites and answer the related questions. Your goal is to gain a better understanding of the carbon, nitrogen and water cycle and to understand the common soil profile. Background: In biogeochemical cycles (including carbon, water and nitrogen cycles), elements are transported between the atmosphere, biosphere (living things), hydrosphere (water), and geosphere (rocks, minerals, and soils). These cycles help us remember that Earth is a complex system. Carbon Cycle: Go to http://www.windows.ucar.edu/tour/link=/earth/Water/co2_cycle.html and answer these questions: 1. Draw the carbon cycle: 2. How does carbon exist in the atmosphere? Carbon exists in the molecule CO2 in the atmosphere. 3. How are fossil fuels created? When plants and animals die, they decay and the carbon from their bodies can become buried underground. After millions of years, the carbon becomes fossil fuels. 4. Describe two ways that carbon enters the atmosphere. Carbon can enter the atmosphere when animals exhale CO2 and when fossil fuels are burned. 5. How are the oceans involved in the carbon cycle? Carbon can dissolve into the ocean water. Organisms living in the oceans can use that carbon to build their skeletons and shells. 6. How is the temperature of the Earth partly controlled by carbon? Carbon is part of CO2 which is a greenhouse gas. Greenhouse gases trap some heat within the Earth’s atmosphere and allow the planet to be warm enough for us to live here. 7. What role do rocks have within the carbon cycle? Over time, carbon can be deposited through the collection of shells/skeletons of organisms. These deposits over long periods of time can become limestone. Go to http://www.windows.ucar.edu/earth/climate/carbon_cycle.html to play the carbon cycle game. You are a carbon atom! 8. Where are you starting within the carbon cycle? You are beginning as carbon that has just been released from the burning of fossil fuels. “Click to begin your journey” 9. How much of the atmosphere is made of carbon dioxide (CO2)? 0.04% of the atmosphere is CO2. 10. By how much has CO2 increased in the atmosphere during the past 150 years? CO2 concentration has increased by 30% during the past 150 years. As you work through this game, take some notes about where you go as a carbon atom. Make sure you visit all reservoirs! The deep ocean When carbon accounts for more How much carbon enters the deep than _____ % of does the surface True or False: ocean, how long the Earth’s ocean absorb from When plants die does it stay there? carbon. the atmosphere and decay, they _______________ each year? bring carbon into ______________ True or False: soil. Phytoplankton are tiny plants and 11. Next stop = _________________________________________ algae that float in What did you learn? the ocean and take up carbon dioxide A.M.V. as they grow. 12. Next stop = _________________________________________ What did you learn? A.M.V. 13. Next stop = _________________________________________ What did you learn? True or False: Plants both absorb CO2 from the atmosphere and release it into the atmosphere. A.M.V. 14. Next stop = _________________________________________ What did you learn? A.M.V. 15. Next stop = _________________________________________ What did you learn? A.M.V. Nitrogen Cycle: Go to: http://www.elmhurst.edu/~chm/onlcourse/chm110/outlines/nitrogencycle.html and answer these questions. 16. What are the two conditions under which nitrogen will react with oxygen? (In other words, what is necessary for nitrogen in the air to combine with oxygen?) Nitrogen will only react with oxygen in the presence of high temperatures and pressures – found near lightning bolts and in combustion reactions in power plants or internal combustion engines. 17. What are the two compounds that are formed when nitrogen combines with oxygen? NO and NO2 18. How does nitric acid (HNO3) form? Nitric acid forms when NO2 reacts with rainwater. 19. Why is nitric acid (HNO3) important? This is the form of nitrogen that can be used by plants as a nutrient. Go to: http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/N/NitrogenCycle.html and answer these questions. 20. What percentage of the air we breathe is nitrogen? Air is 79% nitrogen. 21. Even though considerable nitrogen is available in the air, most plants do not use the nitrogen (N2) found in the air. Why not? Plants cannot use nitrogen in that form. 22. In what compounds can plants use nitrogen? Plants usually get their nitrogen in nitrate ions (NO3-), ammonia (NH3), and urea ((NH2)2CO). 23. How do animals get the nitrogen they need? Animals get their necessary nitrogen by eating plants or other animals. 24. Atmospheric nitrogen (N2) is pretty inert. This means that it does not easily break apart. When molecules do not break apart easily, it is difficult to impossible for organisms to use them as a nutrient source. As a result, nitrogen fixation is the term used to describe the process of breaking up N2. a. What is atmospheric fixation? Lightning can break apart the nitrogen molecule which is then dissolved into rain and washed to the earth. This contributes to 5-8% of all nitrogen fixation. b. What is industrial fixation? [This is how artificial fertilizers are made.] At high pressure and temperature, atmospheric nitrogen (N2) can be combined with hydrogen (from natural gas) to create ammonia (NH3) or urea and ammonium nitrate (NH4NO3) – forms of nitrogen that plants can use. c. What is biological fixation? (In your answer, describe the types of plants associated with the symbiotic relationship.) Some bacteria and archaea are able to fix nitrogen into a usable form for plants. Many of these bacteria live symbiotically with legumes (soybeans, alfalfa). Go to: http://www.physicalgeography.net/fundamentals/9s.html and answer these questions. 25. Draw the nitrogen cycle. (Remember there is another diagram on the previous website that might help you too.) If you’re not sure what a term means, look through the reading and links for help. 26. Why is nitrogen needed by plants and animals? Nitrogen is used for amino acids, proteins and nucleic acids. Water Cycle: Go to: http://observe.arc.nasa.gov/nasa/earth/hydrocycle/hydro2.html and answer these questions. 27. What is condensation? Condensation is the process of water changing from a vapor to a liquid. How do clouds form? As warm air rises, it loses energy, which causes the temperature to drop. The water vapor then changes state from vapor to liquid/ice and creates the cloud. 28. What is precipitation? Precipitation is when water is released from clouds as rain, sleet, snow or hail. 29. What is infiltration? Infiltration is the portion of precipitation that seeps into the ground. What factors (characteristics) affect how much infiltration can occur? The amount of water that infiltrates the soil varies by land slope, type and amount of vegetation, soil type, rock type and moisture content, and the openings in the surface. 30. What is runoff? Runoff is water flowing along the surface. Runoff occurs when water is unable to infiltrate the ground. 31. What is evapotransipiration? Evapotranspiration is the combination of evaporation from the ground and transpiration by plants. This is how water re-enters the atmosphere. What is transpiration? Plants lose water through their leaves in the process of transpiration. 32. Take the Hydrologic Cycle Quiz (There’s a link at the end of the descriptive pages or you can access it through: http://observe.arc.nasa.gov/nasa/earth/hydrocycle/hydroquiz.html) Go to: http://www.educ.uvic.ca/faculty/mroth/438/WEATHER/watercycle.html and use the information provided (and from the previous website) to draw a diagram of the water cycle. 33. Diagram: Soil: Go to: http://library.thinkquest.org/J003195F/soil.htm and answer these questions. 34. How does soil form? It takes a long time (oftentimes 1000s of years) for soil to form. Surface rocks break down into smaller pieces and organic material gets mixed in as vegetation decays. 35. What is weathering? Weathering is the process of breaking down rocks. 36. What is the difference between physical weathering and chemical weathering? In physical weathering the rock material is broken into smaller pieces of the same material, whereas, in chemical weathering the rock material composition is changed. Go to http://library.thinkquest.org/J003195F/soil1.htm or click on the link “Soil Composition” on the above website. 37. What is soil? (Or, what is soil made of?) Soil is a mixture of rocks, minerals, and dead, decaying plants and animals, water and air. Go to: http://www.sccdistrict.com/soilpro.htm and answer these questions. 38. What characteristics help determine the type of soil found in any location? Parent material, climate, living organisms, topography and time influence the specific type of soil that develops in an area. 39. Diagram the soil profile. Include these layers: O horizon, A horizon, B horizon, C Horizon, and Bedrock. You should provide a few characteristics of each horizon. O-Horizon: Organic material – detritus, leaf litter at the surface; it’s dark due to decomposition. A-Horizon: Topsoil – darker, loose with crumbly organic matter, most productive layer B-Horizon: Subsoil – lighter and denser, lower organic material, contains dissolved minerals C-Horizon: transition between soil and parent material; chunks of parent rock and minerals present Bedrock: solid parent material found below the C-horizon 40. Why do the O- and A-Horizons have the darkest color? Commonly the O- and A-horizons are darker in color because of decaying organic material. 41. What happens to minerals and nutrients found in the topsoil as water flows through it? Many soluble minerals and nutrients will dissolve in the water and will leech (move) into lower layers. 42. What does “leach” mean? Leaching means that water is flowing through something.









