(I) Temperature and Thermometers
advertisement

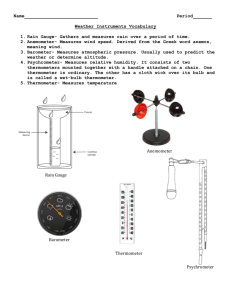
F. 3 Physics--Heat F.3 PHYSICS - HEAT (I) Temperature and Thermometers A. Temperature It is a measure of the degree of hotness or coldness of an object. It is measured by a . B. Temperature scale a To obtain a temperature scale, firstly two fixed points are defined, they are: (i) Lower fixed point (ice point) It is defined as the (ii) Upper fixed point (steam point) It is defined as the temperature of pressure. of pure ice under normal atmospheric pressure. above pure boiling water under normal atmospheric b Then the interval between these two fixed points is divided into different number of divisions according to different temperature scales used. For example: (i) Celsius scale The interval between the two fixed points is divided into Each division is one degree. The unit is degree Celsius (OC). equal divisions. (ii) Kelvin scale (absolute temperature scale) The unit is kelvin (K). Each kelvin has the as a degree Celsius. The lower fixed point is 273 K; the upper fixed point is 373 K. The temperature of 0 K is called . C. Thermometers a. A good thermometer should be , , and . b. There are different types of thermometer. They work on different physical properties called The properties change continuously with temperature. For example: Type of thermometer Liquid-in-glass thermometer Rotary thermometer Thermocouple thermometer Resistance thermometer Thermistor thermometer Thermometric property Matter expands as the temperature is increased. Two different metals expand by different amounts as the temperature is increased. The electric current flowing in a circuit formed by two junctions consisting of two metals increases as the temperature difference between the two junctions is increased. The resistance of a metal coil increases as the temperature is increased. The resistance of a thermistor decreases as the temperature is increased. c. Liquid-in-glass thermometer The following diagram shows the general structure of a liquid-in-glass thermometer. Revised 9/2006 Page 1 of 16 F. 3 Physics--Heat The two common types are . They have different advantages and disadvantages. and (i) Mercury-in-glass thermometer Range: From to Advantages 1. It responds to temperature changes quickly. 2. It has a high boiling point (357 OC). 3. It expands evenly on heating. 4. It does not wet glass. Disadvantages 1. Mercury is a very poisonous substance. 2. It cannot be used in very cold places (freezing point of mercury is -39 OC). (ii) Alcohol-in-glass thermometer Range: From to Advantages 1. It has a low freezing point (-115 OC). 2. It expands evenly on heating. 3. Its expansion is about six times that of mercury. 4. Alcohol is not poisonous. (iii) Clinical thermometer Range: From Disadvantages 1. Alcohol needs to be dyed because it is colourless. 2. It does not respond to temperature changes quickly. 3. It cannot be used in hot places (boiling point of alcohol is 78 OC). to It is a special mercury-in-glass thermometer designed for measuring the temperature of . It only needs to measure a narrow range of temperature from to . There is a very narrow in the tube just above the bulb. When the thermometer is taken out from the user’s mouth, the mercury in the bulb contracts and the mercury column breaks at the and does not go back to the bulb. The temperature of the body can be read. After the temperature has been read, the mercury in the glass tube must be into the bulb before next use. d. Rotary thermometer It consists of a . It can be used to measure temperature from Advantages: Disadvantage: It is used in ovens and large freezers. Revised 9/2006 to . . . Page 2 of 16 F. 3 Physics--Heat D. Examples of calculation – calibration of thermometers 1. The distance between the upper and the lower fixed points on an unmarked thermometer is 23 cm. If the lower fixed point is at the 10 cm mark, what is the temperature corresponding to the 15 cm mark on the Celsius scale? Sketch Method 1 cm o 15 T 10 0 L. F. P. C 100 U. F. P. . 23 Method 2: By graphical method A calibration graph is plotted by using the points ( , ) and ( , (They represent the lower fixed point and the upper fixed point respectively.) Revised 9/2006 ). Page 3 of 16 F. 3 Physics--Heat 2. The resistance of platinum varies with temperature. In a certain resistance thermometer, the resistance of the platinum coil in the thermometer at the lower and the upper fixed points are 2.5 and 3.3 Ω respectively. When the resistance is 3.1 Ω, the temperature measured by a gas thermometer is 80 OC. Find the difference between the two temperatures read by these two thermometers? Sketch Method 1 Method 2: By graphical method A calibration graph is plotted by using the points ( , ) and ( , (They represent the lower fixed point and the upper fixed point respectively.) Revised 9/2006 Ω 3.3 o 3.1 T 2.5 0 L. F. P. C 100 U. F. P. ). Page 4 of 16 F. 3 Physics--Heat (II) Heat and Internal Energy A. Temperature, heat and internal energy a. Temperature Temperature is not a measure of the heat ‘content’ of a body. Temperature is a measure of the degree of hotness or coldness of a body. It is a quantity which measures the the random motion of molecules in the system. due to The higher the temperature of a body is, the its particles move or vibrate. b. Heat Heat should not be confused with internal energy. Heat is not the energy stored in a body, the energy stored in a body is its internal energy. Heat is the energy transferred from one body to another as a result of a temperature difference between the bodies. c. Internal energy The internal energy of a body is the sum of the kinetic energy of random motion and the potential energy of all its particles. When the temperature of a body rises, its particles move or vibrate more vigorously and the average kinetic energy of its particles . When a body changes from solid to liquid or from liquid to gas, its particles become . As a result, the of the particles . In both cases, the of the body increases. An increase in the temperature of a body implies an increase in its internal energy, but the reverse may not be true. For example, when a body melts, the addition of energy to increase the internal energy of the body does not involve the body in a temperature change. B. Increasing the internal energy of a body a. By heating Heating is the process in which energy is transferred from one body to another as a result of a temperature difference between the bodies. When two bodies of different temperatures are touching each other, there will be a energy from the hot body to the cooler body until they reach the temperature. of b. By doing work (p. 29) Work is the energy transferred when a force moves through a distance. Examples of doing work are rubbing a block on a rough surface, hammering a metal object, compressing gases, etc. In all cases, a force is exerted on a body through a distance. Work is defined as: Force and distance are measured in newton (N) and metre (m) respectively, the unit of work is newtonmeter (Nm) or joule (J). In fact, all forms of energy are measured in joule. In all energy transferring processes, energy is neither created nor destroyed. It is only converted from one form to another. This is the principle of conservation of energy. c. By passing a current through a resistance wire By passing a current through a resistance wire, the wire gets hot. This is due to the heating effect of current. Electrical work is done to increase the internal energy of the wire. Revised 9/2006 Page 5 of 16 F. 3 Physics--Heat d. Power Power is the rate of transfer of energy. It shows that how fast a body is heated. It is defined as power = energy / time or p = E/t The unit of power is J s-1 or watt (W). 1W =1Js The energy consumed by an electrical appliance can be measured by a joulemeter or a kilowatt-hour meter. 1 kilowatt-hour (kWh) = 1000 J s-1 x 3600 s = 3.6 x 106 J (or 3.6 MJ) C. Energy transferred by heating Very often we need to calculate how much energy is needed to heat up a substance, for example, to estimate the cost to maintain an indoor heated swimming pool. When water is heated, its temperature rises. The temperature change (ΔT) depends on the and the of the water. We can find out how these quantities are related by using the apparatus shown in the following figure. a. Relation between mass and energy transferred Experiment shows that to produce the same temperature change (ΔT) the energy (E) needed increases with the mass (m) of water. i.e. E m for constant ΔT b. Relation between temperature change and energy transferred Experiment shows that for the same mass (m) of water, large temperature change (ΔT). i.e. ΔT E energy (E) is needed to produce a for constant m Combining the two results, E m ΔT That is, the energy needed to heat up the water is to the mass of water and the temperature change. It is also true for other substances. Mathematically the above equation can be written as: E = k m ΔT k is a constant What is the physical meaning of the constant? To answer this question, we need to define a new term called specific heat capacity. Revised 9/2006 Page 6 of 16 F. 3 Physics--Heat D. Heat capacity and specific heat capacity a. Specific heat capacity (s. h. c.) The specific heat capacity of a substance is the amount of energy that is needed to raise the temperature of 1 kg of the substance by 1 OC (or 1 K). It is designated by the symbol c. Its unit is written as J kg-1 OC-1 or J kg-1 K-1 Since E ∞ m ΔT , the following argument holds: Energy needed to raise the temperature of l kg of substance through 1OC = c joule Energy needed to raise the temperature of m kg of substance through 1OC = c m joule Energy needed to raise the temperature of m kg of substance through ΔT OC = c m ΔT joule Therefore we have the equation E = c m ΔT. Comparing the two equations E = k m Δ T and E = c m ΔT, the constant k is equal to c. Experiment shows that substances have specific heat capacities. (Refer to the table on p. 34.) The the specific heat capacity of a substance is, the the temperature of the substance is changed. b. Heat capacity (h. c.) It is sometimes convenient to ‘lump together’ the quantities m and c in the equation E = c m ΔT. The combined quantity m c is called the heat capacity of the body. It is usually designated by the symbol C. It is then defined as the amount of energy that is needed to raise the temperature of a body by 1OC (or 1 K). Its unit is written as J OC-1 or J K-1. The equation E = c m ΔT then becomes E = C ΔT c. Examples of calculation 1. If 150000 J of energy is transferred to 3 kg of water which initial temperature is 20 OC. Assume that all energy is transferred to the water, what is the final temperature of the water? (Specific heat capacity of water = 4200 J kg-1 OC-1) Given energy transferred, E = 150000 J mass, m = 3 kg initial temperature of water, Ti = 20 oC specific heat capacity of water, c = 4200 J kg -1 oC-1 Revised 9/2006 Page 7 of 16 F. 3 Physics--Heat 2. In an experiment, 0.2 kg of oil was heated from 25 OC to 40 OC. The initial reading on the joulemeter was 22000 J, and the finial reading was 29164 J. Estimate the specific heat capacity of oil. Given energy transferred, E = (29164 – 22000) = 7164 J mass of oil, m = 0.2 kg rise in temperature, ΔT = (40 – 25) = 15 oC 3. An immersion heater takes 2 minutes to raise the temperature of 0.5 kg of water by 50 OC. Calculate the power of the heater. (Specific heat capacity of water = 4200 J kg-1 OC-1). Given time of heating, t = 2 × 60 = 120 s mass of water, m = 0.5 kg rise in temperature of water, ΔT = 50 oC specific heat capacity of water, c = 4200 J kg -1 OC-1 Revised 9/2006 Page 8 of 16 F. 3 Physics--Heat E. Mixture When a hot body 1 at temperature T 1 is in contact with a cooler body 2 at temperature T 2 , finally they reach the same temperature T. It is because internal energy flows from the hot body to the cold body. It is assumed that no energy is lost to the surroundings. According to the principle of conservation of energy (p. 39), the energy lost by the hot body is equal to the energy gained by the cold body. i.e. energy lost by the hot body = energy gained by the cold body m1 c1 (T1 - T) = m2 c2 (T - T2 ) If the energy lost to the surroundings (ΔE) is not negligible, the above equation should be modified as m1 c1 (T1 - T) = m2 c2 (T - T2 ) + Δ E Revised 9/2006 Page 9 of 16 F. 3 Physics--Heat F. Examples of calculation Specific heat capacity of water = 4200 J kg -1 OC-1 1. 0.2 kg of water at 25 OC is added to 0.3 kg of water at 50 OC in a polystyrene cup. What is the temperature of the mixture? Given mass of hot water = 0.3 kg, temperature of hot water = 50 oC mass of cold water = 0.2 kg, temperature of cold water = 25 oC 2. A piece of copper of mass 0.5 kg is heated to 100 OC in boiling water and then put into 0.2 kg of water at 20 O C. If the temperature of the mixture is 35 OC, what is the specific heat capacity of copper? Given mass of copper = 0.5 kg temperature of copper = 100 oC mass of water = 0.2 kg temperature of water = 20 oC temperature of the mixture = 35 oC Revised 9/2006 Page 10 of 16 F. 3 Physics--Heat 3. In a bathroom, there is one hot water tap and one cold water tap. They deliver water at 0.4 kg per second at 70 O C and 0.3 kg per second at 20 OC, respectively. Both taps are turned on for two minutes. (a) What is the temperature of the water in the bath? (b) If a final temperature of 35 OC is wanted, how long should the cold water tap be turned on? (a) rate of hot water delivered = 0.4 kg s-1 temperature of hot water = 70 oC rate of cold water delivered = 0.3 kg s-1 temperature of cold water = 20 oC time of delivery = 2 min = 120 s (b) Given temperature of hot water = 48.6 oC temperature of cold water = 20 oC final temperature of the water = 35 oC From (a), mass of hot water = (0.3 + 0.4) kg s -1 x 120 s = 84 kg Revised 9/2006 Page 11 of 16 F. 3 Physics--Heat (III) Transfer Processes There are three basic means of heat transfer: conduction, convection and radiation. A. Conduction a. It is a process in which heat flows through a medium from places of higher temperature to places of lower temperature. It can occur within a body or between two bodies in contact. The rate of conduction of heat depends on the conductivity of the material, the dimensions of the conductor and the temperature difference between the heat source and the conductor. Metals are good conductors of heat; non-metals are poor conductors (or called insulator) of heat. Heat is conducted faster through a rod if it is short and thick. Air and water are poor conductors of heat. The best heat insulator is a vacuum. b. Microscopic interpretation of conduction The conduction of heat in a non-metal is caused by the collisions between neighbouring molecules. The conduction of heat in a metal mainly depends on its free electrons. In a liquid, the conduction of heat is poor. The molecules in a liquid are not tightly bound with each other. Therefore, the effects of collisions with neighbouring molecules are not as strong as those that occur in a solid. The effects are weakest in a gas, as the molecules are completely separate from each other in this state. c. Applications of good conductors and insulators of heat Good conductors: the exteriors of cooking utensils; heat sinks inside computers, etc. Good insulators: Clothes for cold weather; handles of cooking utensils; the lining inside the walls of refrigerators, etc. d. Practical 3.1 – 3.3 B. Convection a. It is the process that transfers heat by the movement of a fluid from places of higher temperature to places of lower temperature by the movement of the fluid itself. The movement of a fluid when it is heated is explained by the expansion of the fluid. The fluid becomes less dense and is forced upwards by the surrounding, cooler, denser fluid. As a result of this movement, heat is transferred. b. Microscopic interpretation of convection The mass movement of the more energetic molecules in a fluid explains convection. Since the mass movement of these molecules is slowed by their collisions with other molecules, convection is usually less efficient than conduction. c. Daily examples of convection of heat Radiators are usually placed near the floor Heating elements of electric kettles are located near the bottom. Formation of sea breezes and land breezes d. Practical 3.4 Revised 9/2006 Page 12 of 16 F. 3 Physics--Heat C. Radiation a. It is the process that transfers heat from one place to another by means of electromagnetic waves. Radiation is the flow of heat from one place to another by means of electromagnetic waves. Radiation can transfer heat through a vacuum. Infrared radiation produces the greatest heating effect. A hotter object will emit more intense infrared radiation than a cooler one. Dark surfaces are good emitters and good absorbers (or poor reflectors) of radiation, while polished surfaces are poor emitters and poor absorbers (or good reflectors) of radiation. D. Examples involving different heat transfer processes of heat a. Vacuum flask A vacuum flask is a double-walled glass vessel. The space between the glass walls is a vacuum. The facing walls of the evacuated space inside are silvered. The mouth of the flask is covered with a stopper made of good insulator. The stopper and the vacuum between the silvered walls reduce transfer of heat by conduction and convection; the silvered walls reduce transfer of heat by radiation. b. Electric kettle In an electric kettle, a thick metal tube surrounds the heating element near the bottom. Electricity flows through the heating element, the element gets red-hot and radiates energy to the metal tube. Energy is transferred from the metal tube to the water by conduction. A convection current takes place inside the water as the warm water at the bottom rises upwards and spreads outwards near the surface. The air in contact with the sides of the kettle is heated by conduction; the warm air in contact with the kettle rises upwards by convection. This contributes to energy loss. Energy is also radiated away from the sides of the kettle. The major form of energy loss is caused by the evaporation of the water as it is heated up. c. Greenhouse A greenhouse is a glass (or plastic) house in which we keep plants. The roof of the greenhouse allows visible light to pass through, but reflects infrared radiation. The soil and the plants absorb the visible light from the sun and their temperature is raised. They, in turn, emit infrared radiation. The infrared radiation is reflected by the roof and is trapped inside the greenhouse. The temperature inside the greenhouse rises. The roof also reduces heat loss by convection. d. Greenhouse effect The earth resembles a large greenhouse. Carbon dioxide and other gases, such as methane, in the earth’s atmosphere let visible radiation pass, but not infrared radiation. The surface of the earth absorbs most of the visible radiation, before emitting some infrared radiation back into the atmosphere. This radiation is trapped within the atmosphere by these gases. The temperature of the atmosphere rises. Our heavy use of fossil fuels and deforestation contribute to the carbon dioxide build-up; global warming happens. Adverse effects: (i) The polar ice caps will melt and flood the low-lying coastal regions. (ii) Global weather patterns will change and lower crop production. Solutions: (i) To reduce our dependence on fossil fuels. (ii) To increase the plant cover on the earth’s surface. Revised 9/2006 Page 13 of 16 F. 3 Physics--Heat (IV) Change of State A. States of matter a. There are three states of matter: , and . Heating or cooling can change a substance from one state to another. The following diagram summarises the names of these changes. S O L I D G A S Remark: melting point = freezing point boiling point = point of condensation point of condensation = point of sublimation b. The energy absorbed or released by a substance when change of state occurs without a change of temperature is called latent heat of fusion (change of state between solid and liquid), latent heat of vaporization (change of state between liquid and gas) or latent heat of sublimation (change of state between solid and gas). The word ‘latent’ is used because the energy does not change the temperature of the substance. c. The temperature of a substance stays constant when the substance is changing its state, the temperature is called melting point (solid changes to liquid), freezing point (liquid changes to solid), boiling point (liquid changes to gas), condensation point (gas changes to liquid or to solid) or sublimation point (solid changes to gas). B. Cooling curve When a melted substance cools down, the graph of temperature against time is a characteristic curve called cooling curve. The following figure is the cooling curve of octadecan-1-ol, which shows the typical shape of a cooling curve. Revised 9/2006 Page 14 of 16 F. 3 Physics--Heat melting point room temperature The curve can be divided into 3 regions. In region AB, the temperature drops because In region BC, the liquid changes into a solid. There is a mixture of liquid and solid. The temperature . remains constant because the In region CD, solidification is complete, there is . The temperature of the solid then falls. C. Specific latent heat of fusion The specific latent heat of fusion of a substance is the amount of energy (in joule) needed to melt 1 kg of the solid to liquid without changing the temperature. The unit of specific latent heat of fusion is Jkg-1. The energy (E) needed to melt a solid at its melting point can be calculated by the following equation: E = m Lf m: mass Lf: specific latent heat of fusion D. Specific latent heat of vaporization The specific latent heat of vaporization of a substance is the amount of energy (in joule) needed to vaporize 1 kg of the liquid to gas without changing the temperature. The unit of specific latent heat of vaporization is Jkg-1. The energy (E) needed to boil a liquid at its boiling point can be calculated by the following equation: E = m Lv Revised 9/2006 m: mass Lv: specific latent heat of vaporization Page 15 of 16 F. 3 Physics--Heat E. Evaporation It is a process that a liquid changes to a vapour without boiling. It can occur at all temperatures and at the surface only. (While boiling occurs at the boiling point of the substance and throughout the whole volume.) Latent heat of vaporization is absorbed and a cooling effect is achieved. The rate of evaporation increases with a. an increase in ; b. an increase in ; c. an increase in the of across the surface; d. a decrease in ; e. a decrease in atmosphere pressure; F. Application – refrigerator G. Microscopic interpretation of evaporation The molecules in a liquid are to move about. Molecules at the can escape from the liquid if they gain enough kinetic energy from collisions. Only those molecules with kinetic energy have escaped, the kinetic energy of the molecules remaining in the liquid will be and the liquid is cooled down H. Example of calculation The power of a heater is 100 W. It is used to heat 0.2 kg of a solid. The temperature-time graph for the substance is shown in the figure on the right. (i) (ii) (iii) (iv) What is/are the state(s) of the substance in regions A, B and C? What is the melting point of the solid? Calculate the specific heat capacity of the solid? Calculate the specific latent heat of fusion of the substance? Assume that all the energy supplied by the heater is transferred to the substance. Solution: -- End of Heat -- Revised 9/2006 Page 16 of 16


![Applied Heat Transfer [Opens in New Window]](http://s3.studylib.net/store/data/008526779_1-b12564ed87263f3384d65f395321d919-300x300.png)