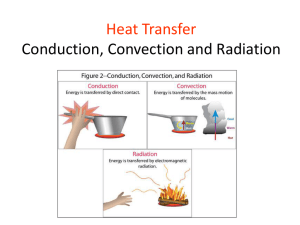
Chapter-10 Temperature and Heat
advertisement

Chapter-10 Temperature and Heat 1 Temperature and First Law of Thermodynamics 2 Heat and Specific Heat Capacity 3 First Law of Thermodynamics 4 Ideal Gas law 5 The Flow of Heat 6 Essay Topic for Final: Global Warming Body Temperature Thermometer Thermometric Property Mercury thermometer Length of mercury column Constant-volume gas thermometer Pressure of the gas Thermocouple Voltage Ear thermometer Infrared radiation The zeroth Law of Thermodynamics Consider three systems A, B, & T. If system A is in thermal equilibrium with system T and system B is in thermal equilibrium with system T, then systems A and B are in thermal equilibrium with each other. Thermometer Standard Temperatures Calibration Temperature Conversion 9 Tf Tc 32 5 Fahrenheit scale Celsius scale 212 100 Unknown temperature Tf Tc Freezing point of water 32 0 Boiling point of water Absolute Zero Temperature Tk Tc 273 Temp. Conversion Problems E1 & E4 9 Tf Tc 32 5 Tk Tc 273 E1: An object has a temperature of 45°C. What is its temperature in °F? E4: The temperature on a warm summer day is 95 degrees F. What is this temperature a. In degrees Celsius? b. In Kelvin? Heat Heat is energy that flows from a higher-temperature object to a lower-temperature object because of the difference in temperatures. Units for Heat SI unit for heat is the joule, J. Calorie is another unit for heat. It comes with a lower case and an upper case. Nutritionists use the word “Calorie,” with a capital C, to specify the energy content of foods. For example, a regular 12-oz can of soda has about 140 Calories. The cgs unit of heat is the calorie, with a lower case. One calorie (1 cal) is defined as the amount of heat needed to raise the temperature of one gram of water by one Celsius degree. 1 food Calorie = 1000 calories = 1 kcal 1 calorie = 4.186 J. Specific Heat Capacity Specific heat capacity of a material is the quantity of heat needed to change a unit mass of the material by a unit change in temperature. It is a property of the material. Specific Heat Capacities of Some Common Substances Substance Water Ice Specific heat capacity [cal/(g. C°)] 1.0 0.49 Steam Ethyl alcohol Steel 0.48 0.58 0.11 Aluminum Lead 0.215 0.0305 Heat Q The heat Q that must be supplied or removed to change the temperature of a substance of mass m by an amount DT is, Q mcDT where c is the specific heat capacity of the substance. Unit for Specific Heat Capacity: SI: J/(kg · C°) cgs: cal/(g. C°) E6: How much heat is required to raise the temperature of 70 g of water from 20°C to 80°C? Calorimetry SP4: A 150-g of a certain metal, initially at 120°C, is dropped into an insulated beaker containing 100 g of water at 20°C. The final temperature of the system is 35°C. Ignore the heat capacity of the beaker. a. How much heat has been transferred to the water from the metal? b. What is the specific heat capacity of the metal? Phase Changes: Latent Heat Latent heat changes the phase of water without changing its temperature. Latent heat of fusion of water = Lf = 80 cal/g. Latent heat of vaporization of water = Lv = 540 cal/g. E8: How much heat must be added to 60-g of ice at 0°C to melt completely? The First Law of Thermodynamics The internal energy of a system changes from an initial value Ui to a final value of Uf due to heat Q and work W: ΔU = Q - W Ideal Gas Law PV =NkT The Flow of Heat Heat can flow via conduction, convection, and radiation. Conduction and Convection When a metal block and a wooden block, both at room temperature, are picked up, the metal block feels cooler, due to conduction of heat. Conduction is the process whereby heat is transferred directly through a material. Convection is the process in which heat is carried from place to place by the bulk movement of a fluid. Thermos Bottle Radiation Radiation is the process in which energy is transferred by means of electromagnetic waves. Heat transfer by radiation can take place through vacuum. This is because electromagnetic waves are involved in radiation and they can propagate through empty space. Q30: Which heat transfer process is responsible when heat flows through a glass windowpane?