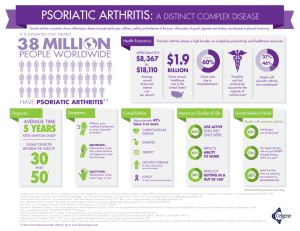
psoriatic arthritis
advertisement

1 D. Gladman PSORIATIC ARTHRITIS Dafna D. Gladman, MD, FRCPC Professor of Medicine University of Toronto Director, Psoriatic Arthritis Program Deputy Director, Centre for Prognosis Studies in The Rheumatic Diseases Toronto Western Hospital, University Health Network Rheumatologist, University Health Network, Mount Sinai Hospital, Consultant Rheumatologist, Sunnybrook Women's College Health Sciences Centre, Toronto, Ontario, Canada Toronto Western Hospital, 399 Bathurst Street MP 1-318, Toronto, Ontario, M5T 2S8. INTRODUCTION Psoriatic arthritis is an inflammatory arthritis, associated with psoriasis (Wright and Moll 1976). Its original definition as seronegative for rheumatoid factor, has been replaced by "usually seronegative" since as many as 15 per cent of the general population, particularly over age 60, may have a positive rheumatoid factor, and rheumatoid factor may be present in more than 10 per cent of patients 2 D. Gladman with psoriasis who do not have arthritis (Gladman et al 1986). The majority of patients with psoriatic arthritis run a benign course. However, in about a fifth of the patients a chronic, progressive, deforming arthritis may develop, resulting in significant joint destruction and limitation of daily activities. EPIDEMIOLOGY Psoriasis is a chronic skin condition which affects 1-3 per cent of the population (Greaves and Weinstein 1995). The association between psoriasis and arthritis might be fortuitous. Since psoriasis is a common condition, and arthritis, particularly osteoarthritis, is quite prevalent, it is conceivable that psoriasis and some unrelated form of arthritis may occur in the same patient. Indeed, some patients with psoriasis do present with a coincidental rheumatoid arthritis, or osteoarthritis. Cats (1990) has argued that psoriasis is just a measure of disease expression in certain patients with peripheral arthritis and spondyloarthropathy. However, epidemiologic evidence described below supports the notion the psoriatic arthritis is a distinct form of arthritis associated with psoriasis. Although the first description of arthritis associated with psoriasis was provided by Aliberti (Eccles and Wright, 1985, O'Neill and Silman, 1994), psoriatic arthritis was considered to be a variant of rheumatoid arthritis until the middle of the 20th Century. Epidemiological studies have confirmed the association 3 D. Gladman between psoriasis and arthritis. These studies have shown an increased frequency of arthritis among patients with psoriasis and an increased prevalence of psoriasis among patients with arthritis. Thus, 6 to 42 per cent of patients with psoriasis may have psoriatic arthritis (Table 1), while the prevalence of arthritis in the general population is about 3 per cent. Likewise, the prevalence of psoriasis among patients with seronegative arthritis is reported to be 20 per cent, while arthritis occurs in only 2 to 3 per cent of the general population (Eccles and Wright, 1985, O'Neill and Silman, 1994). Estimates of the prevalence of psoriatic arthritis range from 0.04% in the Faroe Islands (Lomholt, 1963) to 1.2% in a Swedish study (Helgren, 1969). Lawrence et al (1989) estimated the prevalence of psoriatic arthritis in the US to be 0.67 per cent. A recent report from Olmstead County (Mayo Clinic) suggests a frequency of 0.1% (Shbeeb, et al 2000). This variation may be due to the fact that there are no validated diagnostic or classification criteria for PsA (Gladman, 1995). The discovery of the rheumatoid factor and its association with rheumatoid arthritis helped separate psoriatic arthritis as a distinct entity, since patients with arthritis and psoriasis tended to be seronegative. Radiologic features in psoriatic arthritis were found to be different from those of rheumatoid arthritis (Avila et al 4 D. Gladman 1960). A female preponderance was found in rheumatoid arthritis, whereas the gender ratio among patients with psoriatic arthritis was almost equal (Wright and Moll 1976, Eccles and Wright, 1985, O'Neill and Silman, 1994). Unlike patients with rheumatoid arthritis, patients with psoriatic arthritis may present with a spondyloarthropathy. Psoriatic arthritis is therefore classified with the seronegative spondyloarthropathies. The frequency of psoriatic arthritis has been reported in 6 to 42 per cent of patients with psoriasis (Leczinsky 1948, Little et al 1975, Leonard et al 1978, Green et al 1981, Scarpa et al 1984, Stern 1985, Zanelli et al 1992, Falk and Vandbakk 1993, Barišic-Druško et al 1994, Salvarani et al 1995, Baek et al 2000). Since psoriasis may affect 1 to 3 per cent of the population, and as many of 30 per cent of psoriatic patients may develop psoriatic arthritis, almost 1 per cent of the population may suffer from psoriatic arthritis, which is the expected prevalence of rheumatoid arthritis, and close to the estimated prevalence of 0.67 per cent reported for psoriatic arthritis in the United States (Lawrence et al 1989). Little et al. (1975) and Leonard et al. (1978) suggested that psoriatic arthritis was more common in patients with severe psoriasis. However, psoriatic arthritis may precede the diagnosis of psoriasis in about 15 per cent of the patients (Wright and Moll 1976, Kammer et al 1979, Gladman et al 1987, Jones et al 5 D. Gladman 1994) (Table 2), and the highest prevalence of psoriatic arthritis was recorded among patients attending an outpatient dermatology clinic in Cape Town (Green et al. 1981). Patients with active psoriatic arthritis included in a multicentre drug trial had mild psoriasis and severity of skin and joint disease did not correlate strongly among these 225 patients (Cohen et al, 1999). A cross sectional study of 70 patients with psoriatic arthritis suggested a correlation between the extent of skin and joint severity only among patients with simultaneous onset of skin and joint manifestations (Elkayam et al 2000). CLINICAL FEATURES Psoriatic arthritis affects women and men almost equally, usually in their third or fourth decade (Wright 1956, Kammer et al. 1979, Green et al. 1981, Scarpa et al. 1984, Gladman et al 1987). Nail lesions proved to be the only clinical feature which may identify patients with psoriasis destined to develop arthritis (Gladman et al. 1986). These lesions occur in close to 90 per cent of patients with psoriatic arthritis (Little et al. 1975; Wright and Moll 1976; Kammer et al. 1979; Green et al. 1981; Gladman et al. 1987) and in 46 per cent of patients with psoriasis uncomplicated by arthritis (Gladman et al. 1986). Psoriatic arthritis is inflammatory in nature. It may affect any peripheral joint, as well as the axial skeleton and the sacroiliac joints. Patients usually present 6 D. Gladman with pain, associated with stiffness, which is more marked in the morning, and improves with activity. Morning stiffness of more than 30- min duration is documented in more than half the patients (Gladman et al. 1987). Evidence of inflammation may be detected clinically by the presence of stress pain or joint line tenderness, as well as effusions (Gladman et al. 1990a), although these signs may not be as easily detectable as they are in rheumatoid arthritis, since patients with psoriatic arthritis are less tender than patients with rheumatoid arthritis (Buskila et al. 1992). The inflamed joints in patients with psoriatic arthritis may have a purplish-red discoloration, a feature which is not often seen in rheumatoid arthritis. The effusions in psoriatic arthritis joints tend to be tense, and are often difficult to detect. There is no predilection to particular joints, with the exception of the distal interphalangeal joints. Features which appear to differentiate psoriatic arthritis from rheumatoid arthritis clinically are shown in Table 3. The spondyloarthropathy may present with an inflammatory type of back pain, associated with stiffness and improves with activity. Clinical evidence of sacroiliitis may be obtained by specific tests, including the Gaenslen's manoeuvre, the FABER (Flexion, Abduction, External Rotation of the hip) test and direct pressure over the sacroiliac joints (Gladman et al. 1987; Hanly et al. 1988). Some patients have restricted range of back movements documented by a reduction of 7 D. Gladman flexion-extension as well as lateral flexion and rotation (Gladman et al. 1987; Hanly et al. 1988). Unlike ankylosing spondylitis, many of the patients with psoriatic spondyloarthropathy are asymptomatic, and demonstrate full range of back movement (Gladman et al. 1987; Hanly et al. 1988; Gladman et al. 1992b; Gladman et al. 1993). Clinical Spectrum of psoriatic arthritis In their seminal work Wright and Moll (1976) presented five clinical patterns of psoriatic arthritis: distal arthritis, involving the distal interphalangeal joints (Fig. 1); an asymmetric oligoarthritis involving small or medium sized joints in an asymmetric distribution (Fig. 2); a symmetric polyarthritis, indistinguishable from rheumatoid arthritis; arthritis mutilans, which is a deforming, destructive and disabling form of arthritis (Figs 3 and 4); and a spondyloarthropathy (Figs 5 and 6). Similar descriptions have been reported by others (Kammer et al. 1979; Scarpa et al. 1984; Gladman et al. 1987; Helliwell et al. 1991; Jones et al. 1994; TorreAlonso et al. 1991; Veale et al. 1994b). The frequency of the various patterns has varied in the literature (Table 2). Although initially the most common pattern was thought to be asymmetric oligoarthritis (Wright and Moll 1976), polyarthritis has emerged as the most frequent clinical subset of psoriatic arthritis. In comparison with rheumatoid arthritis, psoriatic arthritis tends to be characterised as an 8 D. Gladman asymmetric form of arthritis. Helliwell et al. (1991) suggested a method for defining symmetrical involvement in patients with psoriatic arthritis, such that for each level of joints if the ratio of the number of matched pairs to the total number of joints was more than 0.5 then the distribution was considered symmetrical. Jones et al. (1994) showed that using this method more patients were found to have a symmetrical arthritis, and Helliwell et al. (2000) found no difference in symmetry between patients with rheumatoid arthritis and psoriatic arthritis. They confirmed that symmetry was a function of the total number of joints involved. Although the distal pattern has been described as typical for psoriatic arthritis, its frequency has varied widely, and some investigators have not been able to identify patients with isolated distal joint involvement. It has also been recognised that the patterns may change with time in individual patients (Gladman 1992). A patient may present initially with an oligoarthritis which later becomes polyarticular, or develop an initial polyarthritis, which persists in only a few joints. Indeed, Jones et al. (1994) documented these changes in pattern over time in over 60 per cent of their patients with psoriatic arthritis. A Spanish study suggested that only two clinical associations could be defined, namely axial arthritis and peripheral arthritis, in a cross-sectional study of psoriatic arthritis (Marsal et al, 1999). Unless radiographs are performed on all patients, joints which had been previously involved may not be identified, and 9 D. Gladman the spondyloarthropathy may be missed (Little et al. 1975; Gladman et al. 1987; Hanly et al. 1988, Battistone et al. 1999). A typical feature of psoriatic arthritis is the development of dactylitis, which presents as a swelling of a whole digit, with inflammation involving distal and proximal interphalangeal, and occasionally the metacarpophalangeal joints (Fig. 7). Dactylitis occurs in over a third of the patients (Gladman et al. 1987), and appears to result from extensive inflammation and effusion in the joints of a particular digit, with an associated tenosynovitis. Ultrasonography demonstrated the presence of both synovitis and tenosynovitis in digits affected by dactylitis (Kane et al, 1999). Tenosynovitis is also a feature of psoriatic arthritis. As in the other spondyloarthropathies, such as Reiter's syndrome and ankylosing spondylitis, Achilles tendinitis, heel pain, and plantar fascitis are common among patients with psoriatic arthritis. Enthesitis, or inflammation at sites of tendon insertion, is frequent, particularly at the Achilles tendon, the insertion of the plantar fascia, and ligamentous insertions around the pelvic bones. These are commonly diagnosed radiologically as spurs (Fig. 8). Recent studies support a role for ultrasonography in identifying involvement of the tendons and entheses in patients with psoriatic arthritis.(Lehtinene et al. 1994; Galluzzo et al 2000). Isolated enthesitis and dactylitis may constitute a specific subset of psoriatic arthritis, even in the absence 10 D. Gladman of peripheral joint involvement (Salvarani et al 1997). McConagle et al (1999) proposed that psoriatic arthritis be considered an enthesopathic disease. The Spondyloarthropathy of psoriatic arthritis The frequency of spinal involvement in psoriatic arthritis has varied from 2 per cent, as isolated back disease, to as high as 78 per cent, when associated with peripheral arthritis (Wright and Moll 1976; Lambert and Wright 1977; Kammer et al. 1979; Scarpa et al. 1984; Gladman et al. 1986; Gladman et al. 1987; Hanly et al. 1988; Moll 1994; Battistone et al. 1999, Baek et al, 2000). Lambert and Wright (1977), found that 40 per cent of 130 patients with psoriatic arthritis had back involvement, based on back pain and reduced spinal mobility. Gladman et al. (1987), documented spinal involvement, based on both clinical and radiological evidence, in 35 per cent of their patients at their first visit to the psoriatic arthritis clinic. This number increased to 51 per cent at follow-up (Gladman et al. 1992b). In both studies patients with spinal involvement tended to be male and older than patients without back involvement. Among patients participating in a multicentre trial who underwent sacroiliac radiography, 78% were found to have at least grade 2 sacroiliitis (Battistone et al. 1999). Among Korean 11 D. Gladman patients with psoriatic arthritis spondyloarthropathy was detected in 50%, and it may even be higher if the patients with psoriasis who had radiographic changes of sacroiliitis were included among the psoriatic arthritis group (Baek et al, 2000). The spondyloarthropathy of patients with psoriatic arthritis is less severe than that seen in ankylosing spondylitis (Hanly et al. 1988; Scarpa et al. 1988; Scarpa 2000). This is evidenced by the lower frequency of symptomatic neck and back disease, as well as less limitation of movement and grade 4 sacroiliitis in patients with psoriatic arthritis compared to those with ankylosing spondylitis (Gladman et al. 1993). Moreover, among patients with psoriatic spondyloarthropathy, there are gender-related differences in disease expression, with more advanced spondyloarthropathy noted among men (Gladman et al. 1992b). The cervical spine in psoriatic arthritis received special attention in two recent studies. Salvarani et al. (1992a) studied 57 patients with psoriatic arthritis, of whom 70 per cent had radiological evidence of cervical spine disease. They identified a high prevalence (23 per cent) of atlanto-axial subluxation. Jenkinson et al. (1994) detected cervical spine disease in 57 per cent of their patients with psoriatic arthritis, of whom only 3 had atlanto-axial subluxation. The majority of their patients had spondylitic type changes with apophysial joint narrowing or fusion and syndesmophytes. In both these studies neck involvement was related to 12 D. Gladman prolonged disease duration. Jeannou et al (1999) found the frequency of neck pain much higher among 30 patients with psoriatic arthritis (73%) than in 30 controls of the same age but who had “common low back pain” (26%). Moreover, among the psoriatic arthritis patients the neck pain was inflammatory in nature in 63.6% compared with only 12.5% of the controls. However, on radiographs none of their subjects had syndesmophytes while 3 patients had atlanto-axial subluxation. EXTRA-ARTICULAR FEATURES Skin psoriasis Skin psoriasis consists of an erythematous scaly area that varies from a localized plaque on the elbows and knees to an incapacitating, generalized skin involvement with significant effect on the cardiovascular and heat regulating mechanism (Goodfield 1994). The skin lesions are classified as: typical psoriasis vulgaris, with major involvement of the extensor surfaces; inverse psoriasis, affecting the flexural areas; pustular psoriasis, which may be localized to the palms and soles, or may be of the more generalized serious form called Von Zambush, and which may pose a threat to life; and the erythrodermic generalized group. The majority of patients with psoriatic arthritis demonstrate the classic psoriasis vulgaris pattern (Wright et al. 1979; Gladman et al. 1986; Gladman et al. 1987). All areas of the skin may be affected, including the mucosa and the nails. Nail lesions include 13 D. Gladman pitting, ridging and onycholysis (Wright and Moll 1976). Two or all of these features in the same patient are in favour of a psoriatic origin for the nail dystrophy (Eastmond and Wright 1979). As already mentioned, nail lesions are particularly common among patients with psoriatic arthritis (Gladman et al 1986). Nail lesions were associated with DIP joint disease in one study (Cohen et al 1999). Other extra-articular features The extra-dermal extra-articular features of psoriatic arthritis are similar to the features described in other seronegative spondyloarthropathies and include iritis, which may occur in 7 per cent of the patients (Gladman et al. 1987). Uveitis associated with psoriatic arthritis was more insidious in onset, posterior, persistent, and more likely to be bilateral than when associated with other spondyloarthropathy (Paiva et al, 2000). Mouth ulcers, urethritis, colitis, and aortic valve disease may also complicate psoriatic arthritis (Wright and Moll 1976). A case of a patient with psoriatic arthritis with pyoderma gangrenosum was described (Smith and White 1994), and we have seen a case in our psoriatic arthritis clinic. The development of lymphoedema of the upper limb in patients with psoriatic arthritis has also been described (Mulherin et al. 1993). Cantini et al. (2001) identified distal extremity swelling with pitting edema in 39/183 (21%) PsA patients and in 18/366 (4.9%) rheumatology clinic controls which excluded spondyloarthropathy 14 D. Gladman (p < 0.0001). They concluded that Upper or lower distal extremity swelling with pitting edema due to tenosynovitis, usually unilateral, is a common feature in PsA patients and may represent the first, isolated manifestation of the disease LABORATORY INVESTIGATIONS IN PSORIATIC ARTHRITIS There are no specific laboratory tests diagnostic for psoriatic arthritis. Anaemia occurred in 14 per cent of the patients presenting to the psoriatic arthritis clinic (Gladman et al. 1987), and at a higher level at follow-up (Gladman et al. 1990b), and was thought to represent the untoward effect of these drugs. Elevated white- cell counts and other acute phase reactants, may also be present (Gladman et al. 1987). Elevated sedimentation rates may be seen in more than 40 per cent of patients with psoriatic arthritis, and likely reflect both joint and skin inflammation (Gladman et al. 1986; Gladman et al. 1987). Hyperuricemia occurred at least once in 20.7% of a cohort of 265 patients with PsA followed prospectively over a 6-year period (Bruce et al, 2000). Although it has been thought to result from the high turnover of skin cells there was no correlation between skin severity and uric acid levels. Since both psoriasis and gout may occur in young males, one must rule out the possibility that the arthritis is crystal induced, before making the diagnosis of psoriatic arthritis in a patient with psoriasis. On the other hand, the presence of an 15 D. Gladman acute monoarthritis, even in the first metatarsophalangeal joint in the presence of psoriasis does not mean the patient has gout. In both these situations a careful search for negatively birefringent, uric acid crystal should be carried out on the fluid obtained by joint aspiration. Patients with psoriatic arthritis are usually seronegative for rheumatoid factor. However, in each series of patients with psoriatic arthritis there are about 10 to 15 per cent of the patients who have a positive rheumatoid factor, albeit in a low titre. In addition, patients with psoriasis uncomplicated by arthritis demonstrate the same frequency of positive rheumatoid factor, despite the fact that they are younger on average than the patients with psoriatic arthritis (Gladman et al. 1986). Antinuclear factor has also been demonstrated in the sera of patients with uncomplicated psoriasis and patients with psoriatic arthritis, in the same frequency (Gladman et al. 1986). Whether this antinuclear antibody reflects the presence of antibodies to stratum corneum antigens is unclear (Gladman 1985). RADIOLOGIC FEATURES OF PSORIATIC ARTHRITIS Radiologic abnormalities may be seen in both peripheral joints and the axial skeleton in patients with psoriatic arthritis (Resnick and Niwayama 1981). The features commonly associated with psoriatic arthritis and which help differentiate it from rheumatoid arthritis include: absence of juxta-articular osteoporosis; the 16 D. Gladman predilection for distal interaphlangeal joints; "whittling" (lysis) of terminal phalanges (Fig. 9); lack of symmetry; gross destruction of isolated joint; "pencil-incup" appearance (Fig. 10); ankylosis (Fig. 10); fluffy periostitis (Fig. 11); both classical and atypical spondylitis (Wright and Moll 1971; Resnick and Niwayama 1981; Fig 5, 6 and 7). However, a direct comparison of radiographs of patients with rheumatoid arthritis and patients with psoriatic arthritis matched for age and disease duration, the differences were not obvious (Rahman et al, 2001). DIAGNOSIS OF PSORIATIC ARTHRITIS There are no available diagnostic criteria for psoriatic arthritis (Gladman 1995). The European Spondyloarthropathy Study Group preliminary criteria for the classification of the spondyloarthropathies were only 65% sensitive for psoriatic arthritis (Salvarani et al 1995). A new classification based on a comparison of clinical and laboratory features of 100 patients with psoriatic arthritis, 80 patients with ankylosing spondylitis and 80 patients with rheumatoid arthritis identified 9 criteria which were 95% sensitive and 98% specific for psoriatic arthritis in the study population (Fournie et al, 1998). These criteria include the presence of psoriasis either in the patient or a relative, distal joint arthritis, inflammatory spinal involvement, asymmetric oligoarthritis, enthesitis, 17 D. Gladman the presence of one of 5 radiological digit criteria, the presence of a relevant HLA antigen and a negative rheumatoid factor. The proposed criteria would be difficult to achieve at the bedside as they require both laboratory and radiological evidence. Moreover, the criteria still require confirmation in an additional, larger patient cohort. None the less, the diagnosis of psoriatic arthritis is generally based on the definition of the disease: an inflammatory arthritis in the presence of psoriatic skin lesions, usually seronegative for rheumatoid factor. In a patient with psoriasis, the development of an inflammatory arthritis makes the diagnosis easier. The clinical and radiologic features described above help identify the patient with psoriatic arthritis who had not previously demonstrated skin lesions. Thus, a patient who presents with an asymmetric oligoarthritis, or an inflammatory polyarthritis which includes distal interphalangeal joints, or peripheral arthritis with a spondyloarthropathy, should be investigated for the presence of psoriasis, and psoriatic arthritis should clearly be considered in the differential diagnosis. The presence of dactylitis is certainly helpful, as is the presence of enthesitis. The skin lesions may be minimal, and indeed "hidden". One must therefore search for these lesions, particularly in the umbilical area, the anal cleft, the groin, the scalp, and the ears. Nail lesions are not always recognised by the patient, and should be 18 D. Gladman looked for carefully. The common occurrence of distal joint involvement means that psoriatic arthritis needs to be differentiated from osteoarthritis. The distal interphalangeal lesions in patients with psoriatic arthritis are inflammatory in nature, and tend to be swollen, such that for the most part they can be differentiated clinically as softer than the hard bony enlargement of Heberden's nodes. The presence of more proximal joint involvement, particularly the wrist and metaacrpophalangeal joints also helps distinguish psoriatic arthritis from osteoarthritis. However, osteoarthritis is a common condition, particularly with advancing age, and a patient may have Heberden's nodes complicating preexisting psoriatic arthritis. Reiter's disease occasionally presents a diagnostic difficulty. The skin lesions in pustular psoriasis may be indistinguishable both clinically and pathologically from those of Reiter's syndrome, and the clinical features of the arthritis and the spondyloarthropathy are similar. Psoriatic arthritis tends to be polyarticular, which may help. Iritis and mucous membrane lesions may be more common in Reiter's disease. PATHOGENESIS OF PSORIATIC ARTHRITIS 19 D. Gladman Although the exact pathogenesis of psoriatic arthritis remain to be elucidated, factors thought to be important include environmental genetic, and immunologic ((Gladman 1992; Abu-Shakra and Gladman 1994). Genetic factors A family history of the skin or joint disease in first-degree family members is obtained in more than 40 percent of patients with psoriatic arthritis (Gladman et al. 1986; Gladman et al. 1987). Moll and Wright (1973) identified the prevalence of psoriatic arthritis among first degree relatives of 88 probands with the disease at 5.5% compared to the calculated prevalence in the UK population of 0.1%. Population based studies and twin studies support the genetic contribution in psoriasis (Espinoza 1985; Eastmond 1994). Segregation studies in psoriasis conclude that a polygenic or a multifactorial pattern is the most likely mode of inheritance (Bhalerao and Bowcock 1998). Population studies in psoriasis revealed an increased frequency of HLA antigens B13, B17, B37, Cw6 and DR7 (Espinoza 1985; Gladman et al. 1986; Eastmond 1994). In psoriatic arthritis, increased frequencies of HLA-B13, B17, B27, B38, B39, DR4 and DR7 have been reported (Espinoza 1985; Gladman et al. 20 D. Gladman 1986; Sakkas et al. 1990). Gladman et al. (1986) compared 158 patients with psoriatic arthritis to 101 patients with uncomplicated psoriasis. They found that the HLA-B7 or B27 antigen were more common among patients with psoriatic arthritis, whereas B17, Cw6 and DR7 were more common among patients with uncomplicated psoriasis. HLA-B27 has clearly been associated with back disease in psoriatic arthritis, thus lending further credence to its grouping with the HLA-B27associated spondyloarthropathy. HLA-DR4 appears to be associated with the peripheral articular pattern of psoriatic arthritis (Gladman et al. 1986). No specific T-cell receptor genes unique to the disease were identified (Sakkas et al.1990), but immunoglobulin heavy chain gene (IgH) on chromosome 14q32 may confer susceptibility to arthritis in patients with psoriasis (Sakkas et al. 1991). Several genetic loci have been identified in family investigations in psoriasis, including loci on chromosome 17q (Tomfohrdre et al. 1994; Nair et al. 1997), 4q (Matthews et al. 1996) and 6p (Burden et al. 1996; Trembath et al. 1997; Leder et al. 1998; Samuelsson et al. 1999; Veal et al. 2001), and 1p (Veal et al. 2001). The strongest association by far is with loci on chromosome 6p. An analysis of all families for whom complete data was available in the literature suggests that HLA-B locus is in linkage disequilibrium with the PSORS1 gene (Leder et al. 1998). However, the exact location and nature of 21 D. Gladman PSOR1 on 6p is unclear. Similar studies are currently underway for psoriatic arthritis. Environmental Factors Infection Support for the role of bacterial antigens in the pathogenesis of psoriasis and psoriatic arthritis comes from indirect observations of enhanced humoral and cellular immunity to Gram-positive bacteria typically found in the psoriatic plaques (Vasey 1985). However, psoriatic plaques often get secondarily infected, thus the cause-effect relationship of bacteria and psoriasis is complicated. Moreover, investigators have demonstrated that there is no specific role for streptococcal responsive synovial T lymphocytes psoriatic arthritis (Grinlinton et al. 1993, Thomssen et al. 2000). However, Prinz (2001) recently pointed out the possibility that an infectious agent may have triggered the psoriatic process, and the immunological response seen in patients with both psoriasis and psoriatic arthritis may be the result of molecular mimcry between streptococcal antigens and epidermal autoantigens. The exacerbation of psoriasis and psoriatic arthritis seen in the context of acquired immunodeficiency virus infection is intriguing (Vasey et al. 1989). The 22 D. Gladman possibility that psoriatic arthritis might be virus induced has been proposed (Luxembourg et al. 1987). Hepatitis C virus has been detected more commonly among patients with psoriatic arthritis, but its role in the pathogenesis of the disease cannot be confirmed (Taglione et al, 1999). Trauma In almost all accounts of psoriatic arthritis there are reports of patients whose arthritis developed after trauma. However, the majority of the reports are anecdotal case reports (Punzi et al., 1998). A case series was reported Scarpa et al. (1992) who found that trauma of some type preceded the diagnosis of psoriatic arthritis in 9 per cent of the cases, whereas in rheumatoid arthritis it was found in only 1 per cent of the patients, suggesting a role for trauma in some patients with psoriatic arthritis. Whether this represents an internal Koebner phenomenon (the appearance of psoriasis at sites of cutaneous injury due to trauma) is unclear. However, substance P, a neuropeptide, and vasoactive intestinal peptide are over expressed in psoriatic skin lesions and in psoriatic synovium (Veale et al. 1993a) Immunological mechanisms 23 D. Gladman The clinical and pathological features of both psoriasis and psoriatic arthritis support the role of immunological factors in the pathogenesis of these conditions. The inflammatory nature of the disease, the cellular infiltrates seen both in skin and joint lesions, and the deposition of immunoglobulins in the epidermis as well as the synovial membrane, all support an immune mechanism (Panayi 1994). Psoriasis is characterised histologically by keratinocyte proliferation, vascular changes and the presence of T Lymphocytes in affected skin (Gottlieb 1988). T lymphocytes, particularly CD8+ cells, are thought to play a prime role in its pathogenesis. Activated T cells have been noted in the affected tissues (both skin and joints) in psoriatic arthritis by most investigators (Gladman 1993; Veale et al. 1993b; Panayi 1994; Veale et al. 1994a). In an animal model, a severe combined immunodeficient (SCID) mouse with grafted unaffected human skin, injection of activated T cells from affected individuals causes psoriasis to develop in the grafted and surrounding skin (Wrone-Smith and Nickoloff 1996). A predominance of CD8+ T lymphocytes with clonal expansion was documented in the synovial fluid from patients with psoriatic arthritis (Costello et al, 1999). Examination of T cell antigen receptor beta chain variable (TCRßV) gene repertoires in skin and synovium reveal common expansions in both sites, suggesting that a common antigen may act as a trigger for disease in both tissues (Tassiulas et al. 1999). PsA synovial explants 24 D. Gladman produced more IL-1, IL-2, IL-10, IFN- and TNF- than those of rheumatoid arthritis or osteroartrhitis. Levels of IL-1, IFN-, IL-10 were higher in synovial tissue than dermal plaques from patients with PsA (Ritchlin et al., 1998). Thus cytokines secreted from activated T cells and other mononuclear proinflammatory cells induce proliferation and activation of synovial and epidermal fibroblasts, leading to the fibrosis reported in patients with longstanding psoriatic arthritis. This is also consistent with the prominent ankylosis seen both clinically and radiologically in patients with psoriatic arthritis. The effect of anti-cytokine therapy further supports the role of cytokines in this disease. Anti-TNF agents have been shown to work in both psoriasis (Chaudhari et al. 2001) and psoriatic arthritis (Mease et al, 2000) The role of metabolites of arachidonic acid, such as prostaglandins and particularly leukotrienes, in the pathogenesis of both psoriasis and psoriatic arthritis has been proposed. Increased levels of Leukotriene B4 in the psoriatic skin lesions have been noted, and injections of this compound has caused intraepidermal microabscesses. Greaves and Camp (1988) proposed an integrated approach to inflammation of human skin, considering both the lipoxygenase system, platelet activating factor and cytokines. However, studies in both psoriasis (Soyland et al, 25 D. Gladman 1993) and psoriatic arthritis (Veale et al. 1994c) failed to demonstrate clinical improvement in either skin or joint manifestations in patients treated with fish oil. TREATMENT OF PSORIATIC ARTHRITIS The treatment modalities employed in psoriatic arthritis are in part based on the pathogenetic mechanisms discussed above. These are based on control of inflammation, and an attempt to modify the immunological mechanisms thought to be operating in this disease (Gladman and Brockbank 2000). The treatment of psoriasis The treatment of psoriatic arthritis includes treatment for the skin condition as well as treatment for the joint disease. The skin lesions are treated by topical medications, aimed at controlling the inflammation and skin proliferation, including tar, anthralin and corticosteroids. In refractory cases, systemic medications such as methotrexate PUVA (psoralen and ultraviolet A light), retinoic acid derivatives and more recently cyclosporin are used (Greaves and Weinstein 1995). The treatment of psoriatic arthritis Non steroidal anti-inflammatory therapy 26 D. Gladman The initial treatment for psoriatic arthritis consists of non-steroidal antiinflammatory drugs (NSAIDs), including enteric-coated acetylsalicylic acid (ECASA), ibuprofen, naproxen, indomethacin, tolmetin, piroxicam, diclofenac sodium and other NSAIDs (Gladman and Brockbank 2000). In patients with significant peripheral arthritis, medications such as ECASA, ibuprofen, naproxen or diclofenac sodium might be preferred. However, for the spondyloarthropathy, indomethacin or tolmetin would be more appropriate. The latter two drugs would also be appropriate if morning stiffness is prolonged, and may be used in conjunction with other NSAIDs. Several of the NSAIDs have been incriminated in exacerbating the psoriasis, perhaps through the prostaglandin mechanism. It may therefore be necessary to change medications if an exacerbation of psoriasis occurs. The newer cycloxygenase-2 (COX-2) selective inhibitors recently released for the treatment of rheumatoid arthritis and osteoarthritis, including celecoxib, rofeocoxib and mobicoxib have not been adequately tested in psoriatic arthritis, and do not appear to be any more effective that the previously available NSAIDs. It remains to be determined whether they have an adverse effect on the skin lesions. Disease modifying drugs for psoriatic arthritis 27 D. Gladman If the arthritis persists despite the use of non-steroidal anti-inflammatory medications, disease-modifying anti-rheumatic drugs are added. Gold Gold has been studied in a controlled fashion in psoriatic arthritis, using either intramuscular (Dowart et al. 1978) or oral (Carrett and Calin 1989) preparations. Intramuscular preparation has been proven superior to the oral gold in patients with psoriatic arthritis (Palit et al. 1990). However, although gold may control the inflammatory process in patients with psoriatic arthritis, it has not prevented progression of erosive disease over a 2-year period (Mader et al. 1995). Penicillamine Penicillamine has also been used successfully in psoriatic arthritis (Roux et al. 1979). Both gold and penicillamine are quite slow acting, however, requiring at least 6 months for a therapeutic effect. Therefore, other medications, whose onset of action is faster have been tried. Antimalarials Although physicians have been reluctant to use antimalarials because of anecdotal reports of flares of psoriasis, and despite the lack of controlled trials, both chloroquine phosphate and hydroxychloroquine have been used (Kammer et al. 1979). Indeed, chloroquine has been shown to reduce disease activity in patients 28 D. Gladman with psoriatic arthritis over a period of 6 months, and the frequency of psoriatic flares was no greater than that observed in the control group (Gladman et al. 1992a). Methotrexate Methotrexate, which has been found to be effective in controlling the skin psoriasis, has been used in psoriatic arthritis since 1964, when Black et al. (1964), performed a double-blind study of 21 patients using parenteral methotrexate. There have been two controlled trials using low-dose weekly methotrexate in psoriatic arthritis. Willkens et al. (1984) demonstrated improvement in grip strength, morning stiffness and joint count in patients with psoriatic arthritis, and physician global assessment was improved in the methotrexate group at 3 months, but only physician global assessment was significantly higher in the methotrexate group compared with the placebo. Zacharia and Zacharia (1987) found significant improvement in pain and functional scores as well as a decrease in the erytherocyte sedimentation rate during treatment with low-dose weekly methotrexate for psoriatic arthritis. Espinoza et al. (1992), in a retrospective uncontrolled study of 40 patients with psoriatic arthritis treated with a mean of dose of 11.2 mg/week of methotrexate during a mean period of 34 months, found that 37 per cent of the patients had an excellent response (no evidence of active synovitis) while 58 per cent had a good 29 D. Gladman response (no more than 4 active joints and a decrease of at least 50 per cent in the number of those previously involved). Only two patients had discontinued the drug because of toxicity; one leucopenia and the other stomatitis. Eleven patients developed liver test abnormalities, however, cirrhosis related to methotrexate was not noted. Abu-shakra et al. (1995) found that while methotrexate reduced the actively inflamed joint count in patients with psoriatic arthritis, it did not prevent disease progression in these patients over a period of 2 years of treatment. None the less, methotrexate is used regularly for the treatment of psoriatic arthritis, particularly in the face of severe psoriasis. Methotrexate has an advantage over gold and penicillamine since it is effective within a few weeks. In addition, because it is given as an intermittent dose, once a week, patients prefer to take it over medications which are required daily, and often in repeated doses. Concerns about severe liver disease resulting from methotrexate therapy which resulted from reports in the late 1960s and early 1970s seem to have been alleviated since more judicious use of intermittent dose has become commonplace. The use of either folic acid or folinic acid has also reduced some of the side effects of methotrexate. Sulphasalazine Sulphasalazine has been shown in several double-blind placebo-controlled trials to be effective in psoriatic arthritis (Farr et al 1990.; Fraser et al. 1993, Combe et al. 30 D. Gladman 1996; Clegg et al. 1998). However, the effect has been modest (Jones et al 2000). A recent study in an outpatient clinic reported that 44% of the patients who started on Sulphasalzine were unable to tolerate it, and that there was no long-term advantage in terms of erosive disease (Rahman et al. 1998). Azathioprine Azathioprine has also been used in psoriatic arthritis (Levy et al. 1972, Lee et al. 2001), but since its effect on the psoriasis is not well recognized, it has not been as useful as it is in rheumatoid arthritis. The use of azathioprine in patients with psoriatic arthritis who had not responded or were unable to tolerate methotrexate was recently described (Lee et al. 2001), with encouraging results. However, compared to other medications used azathioprine was not found to have an advantage in preventing damage in patients with psoriatic arthritis. Retinoids Retinoids have only been studied in uncontrolled fashion, since it is difficult to blind both patients and observers to their side effects (Klinkhoff et al. 1989). While these drugs may be effective against both skin and joint manifestations, their toxicity appears high. Cyclosporin A 31 D. Gladman Cyclosporin A has been studied as a therapeutic option for both psoriasis and psoriatic arthritis (Gupta et. al 1989, Salvarani et al. 1992b). In a comparative study with methotrexate there was significant improvement in the skin and joints of both groups, with no significant difference between them. However there was a higher withdrawal rate in those patients on cyclosporin (Spadaro et al 1995). Although it has been suggested that it is effective and safe, NSAIDs cannot be used concomitantly. Moreover, its adverse effects, particularly on the kidney, preclude its wide spread use. Steroids Oral steroids are usually avoided in psoriatic arthritis, since upon dose reduction they can cause significant flares of the skin psoriasis (Griffith 1997). However, intra-articular steroids may be used at any time, especially when there is a joint which is particularly inflamed. We tend to avoid injecting joints which are surrounded by psoriatic plaques because of fear of causing infections. Anti-TNF agents in psoriatic arthritis The development of anti-TNF agents for the treatment of rheumatoid arthritis which was based on a proposed role for TNF in the pathogenesis of the inflammatory process opened up new avenues for the treatment of both skin and joint manifestations of psoriatic arthritis. Etanercept is a recombinant human protein, 32 D. Gladman consisting of two TNF receptor p75 fused with the FC domain of human IgG1. Consequently it binds TNF and inactivates it. A double-blind controlled trial of 60 patients with psoriatic arthritis, half of whom were also on methotrexate demonstrated an remarkable improvement in the psoriatic arthritis response criteria (87% compared to 27% in the placebo group) which was much better than in the study of sulfasalazine in psoriatic arthritis for which the criteria were developed (Clegg et al 1998; Mease et al 2000). A follow-up open-label study further demonstrated the efficacy of etanercept in patients who were randomized to placebo in the original trial. In this group 65% responded to etanercept. A larger multicentre randomized controlled trial of etanercept in psoriatic arthritis is currently being completed. Etanercept is given subcutaneously at a dose of 25 mg twice a week. Patients can self inject. It is relatively safe, with injection site reactions being the commonest side effects. However, there is a concern about susceptibility to infection. Infliximab is a human/mouse chimeric anti-TNF- antibody that blocks TNF from reaching its receptor. It has also been proven effective in rheumatoid arthritis. Infliximab is also given parentrally, however, it is given by infusion which is more difficult for the patients since they require to attend a medical centre at 6-8 week interval for at least 2 hours. Infliximab was recently demonstrated to be effective in the treatment of severe psoriasis 33 D. Gladman (Chaudhari et al. 2001). There are few case series of its use in psoriatic arthritis. It was given to 10 patients in Germany with good response (A&R 2000). We have had an opportunity to treat 15 patients with severe psoriatic arthritis with infliximab. Seven of the 15 demonstrated greater than 70% reduction in the number of actively inflamed joints. The majority demonstrated excellent response of their skin. However, 5 patients had to discontinue the drug because of adverse effects, which included infection, rectal bleeding, and allergic reactions. Infliximab has been associated with allergic reactions that are thought to result from antibody formation to the foreign protein. Recently there has been concern about reactivation of pulmonary tuberculosis by anti-TNF agents. It is possible that newer anti-TNF agents such as a completely human anti-TNF antibody and TNF receptor to p55 as well as p75 will be available for the treatment of psoriatic arthritis. There are other biologic agents currently under study for psoriasis, including an anti-CD11 antibody, CTLA4-Ig. They have yet to be investigated for psoriatic arthritis. Dietary modification 34 D. Gladman Based on the proposed pathogenesis of both psoriasis and psoriatic arthritis, there may be a role for fish oil preparations or specific immunomodulators in the treatment of both psoriasis and psoriatic arthritis. However, a placebo controlled trial of Efamol marine demonstrated no efficacy in psoriatic arthritis (Veale et al. 1994c). The use of oral vitamin D3 for the treatment of psoriatic arthritis has also been proposed (Huckins et al. 1990). Other medications Psoralen and ultraviolet A light has been used in psoriatic arthritis with some success (Perlman et al. 1979). More recently, the use of extracorporeal photochemotherapy has been proposed (de Misa et al. 1994). Buskila et al. (1991) described a woman with psoriatic arthritis, who experienced a remarkable improvement of her arthritis while she was taking bromocriptine for primary infertility due to hyperprolactinemia. Others have also reported similar results. Peptide T has also been advocated for the treatment of psoriatic arthritis, but information is currently available only from case reports (Abu-Shakra and Gladman 1993). Physiotherapy and occupational therapeutic modalities should be used both for symptomatic relief and to avoid development of deformities. Patients may require splints, and need to be instructed as to energy conservation and joint 35 D. Gladman protection. In patients with the spondyloarthropathy specific back exercises may be necessary. Surgery Surgery is reserved for patients whose joints have become deformed and damaged. Of 444 patients with psoriatic arthritis followed prospectively 31 (7%) had musculoskeletal surgery at an average of 13 years after the onset of joint disease. A high number of actively inflamed joints and advanced radiological damage at first assessment were highly predictive of subsequent surgery (Zangger et al 1998). Seventy-one procedures were performed in 43 patients at one centre during a 10 year period (Zangger et al. 2000). Among patients with polyarticular disease a variety of procedures were performed including complex hand and foot surgery, hip replacements and fusion of individual joints, whereas patients with oligoarticular disease tended to have hip and knee replacements. Four procedures led to complications: an infection in a hip arthroplasty which required removal of the prosthesis, a malunion of an ankle arthrodesis which required correction, a lack of fixation in a PIP joint which was corrected within 12 days, and a death from an undiagnosed hepatic hemangioma. An approach to the treatment of temporomandibular joint disease in psoriatic arthritis has been described (Peterson 36 D. Gladman and Shepherd 1992). Hicken et al. (1994) reported their experience of 27 forefoot and toe arthroplasty or arthrodesis procedures in 17 patients with psoriatic arthritis collected over a 15-year period. The operations were considered successful in 89 per cent of the cases but they did not use standardized methods for assessing functional outcome. Complications occurred in a patient who had a local infection associated with a local flare of psoriasis and required corrective surgery, another patient who required an additional procedure, and a third patient who had delayed union. THE COURSE AND PROGNOSIS OF PSORIATIC ARTHRITIS The course of psoriatic arthritis is variable. There are patients who have few episodes and who recover completely (Gladman et al 2001), but in many the disease is persistent. Roberts et al. (1976) concluded in their follow-up study that "apart from the deforming group the arthritis was not notably progressive". However, there was time lost from work in at least 60 per cent of all patients, and radiologic progression was recorded in about 15 per cent. Stern (1985) noted that more than 50 per cent of the patients with psoriatic arthritis had some limitation on their daily activities, and they were twice as likely to be unemployed as patients with other joint disease. Hanly et al. (1988) and Gladman et al. (1990b), suggested that the disease is progressive, based on the increased number of deformed and damaged 37 D. Gladman joints observed in their patients, who were followed according to a standard protocol in the University of Toronto Psoriatic Arthritis Clinic, where clinical measures of inflammatory activity as well as damage have been validated (Gladman et al. 1990a). Coulton et al. (1989) reported on the outcome of 40 patients hospitalised for psoriatic arthritis who were followed for a mean of 8 years. At the end of that period, none of the patients died. However, 35 per cent of their patients were in Steinbrocker's classes III and IV, supporting the notion that a proportion of patients with psoriatic arthritis become disabled. Clear evidence of progression of deformities was demonstrable when patients were compared at presentation and at follow-up based on duration of follow-up at the psoriatic arthritis clinic (Gladman 1994). Gladman et al. (1995) studied 305 patients who entered the psoriatic arthritis clinic with less than ten deformed joints, and identified clinical indicators for progression through four stages: no deformities, one to four deformities, five to nine deformities and ten or more deformities. Patients who had five or more effused joints at presentation to the clinic were more likely to progress, as were patients treated with disease-modifying drugs, while patients who had a low erythrocyte sedimentation rate were less likely to develop more deformities during follow-up. There was no correlation with disease duration. Moreover, Gladman and Farewell (1995) demonstrated that the HLA antigens B27 in the presence of HLA-DR7, 38 D. Gladman HLA-B39 and HLA-DQw3 in the absence of HLA-DR7 were more important than the clinical features in predicting such progression. The same investigators further demonstrated that the number of actively inflamed joint at each visit is an important predictor of subsequent clinical damage in patients with psoriatic arthritis (Gladman and Farewell 1999). Psoriatic arthritis may no longer be considered a mild form of arthritis. Patients with psoriatic arthritis have an increased mortality risk compared to the general population (Wong et al 1997). While the causes of death are similar to those of the population at large, previously active and severe disease are predictors for early mortality among patients with psoriatic arthritis (Gladman et al. 1998). None the less, the arthritis specific Health Assessment Questionnaire (HAQ) and the generic Medical Outcome Survey Short Form 36 (SF-36) in patients with psoriatic arthritis does not give the same high scores seen in rheumatoid arthritis (Jones et al. 1994; Blackmore et al. 1995; Husted et al 2001). This may very well be due to the fact that the questionnaires correlate highly with pain, which is less likely to be an issue for patients with psoriatic arthritis than for patients with rheumatoid arthritis (Buskila et al. 1992). However, the quality of life in patients with psoriatic arthritis was found to be lower than in patients with uncomplicated psoriasis (Lundberg et al. 2000). 39 D. Gladman Approach to the Management of Psoriatic Arthritis My overall approach to a patient with psoriatic arthritis includes confirming the diagnosis, assessing the extent of disease activity in terms of both joint and skin disease, and assessing the degree of damage that has occurred. This involves a careful history, physical examination, laboratory assessment and radiological evaluation. The goal of treatment is control of inflammation which will hopefully lead to control of symptoms, and prevention of deformities and damage, so that the patient may continue to lead active and productive life. Once the diagnosis has been made and the patient assessed, my approach to management begins with patient education. I explain to the patient that he/she suffers from an inflammatory arthritis, that the treatment is aimed at controlling inflammation and therefore medications need to be taken regularly. The role of daily stresses on exacerbations of both skin and joint disease is reviewed. The need to treat both skin and joint manifestations of the disease is also discussed. These topics often need to be repeated during follow-up. The actual therapeutic approach is then tailored to the individual patient (Box 1). A patient who has mild disease, with minimal skin lesions and mild arthritis without deformities, is treated with topical ointments for the skin, and non- 40 D. Gladman steroidal anti-inflammatory drugs for the joints. I tend to use enteric-coated acetylsalicylicacid, ibuprofen, naproxen or diclofenac for polyarticular disease, and those that complain of pain. In patients with oligoarticular disease, and those with spondyloarthropathies, I tend to use indomethacin or tolmetin. While there is no scientific proof to support this approach, it seems to be empirically correct. However, since patients vary both in their response to and tolerance of different NSAIDS, this sequence is often changed. For individuals who clearly express aversion to taking pills, I tend to choose those NSAIDS which can be given once daily. The selective COX-2 inhibitors may provide gastroprotection, but are not superior to the other NSAIDs in their anti-inflammatory activity. I also use intra-articular corticosteroid injections for individual joints. I find that in psoriatic arthritis the inflammation is intense and deformity can ensue rapidly. My patients are educated to call immediately when a red hot joint appears, and to present themselves for joint injections. Although some people feel that intraarticular steroids are not as effective in seronegative disease as they are in rheumatoid arthritis, this has not been my experience. We have been able to control severe inflammation and prevent damage in joints which we injected early (as judged by what happened to other joints in the same individual). This applies to both large and small joints in this disease. I use joint injections as an adjunct to 41 D. Gladman systemic therapy as well, at any point in the disease, provided the joint is not completely destroyed. In a patient with active and severe arthritis, I use second line medications early. I have used antimalarials, sulphasalazine, methotrexate, gold and azathioprine, for this type of patient. If the patient demonstrates a spondyloarthropathy, I would tend to choose sulphasalazine first, since it seems to control spinal disease as well as peripheral disease, whereas the other medications have not been shown to be as effective for spinal involvement. More recently we have been able to test the ability of the anti-TNF agents to control psoriatic arthritis, and had these drugs been more easily available and accessible I would prefer to use them as early as possible in patients presenting with highly inflammatory disease. In a patient who has severe psoriasis, even if the arthritis is not that severe, I tend to start with methotrexate, since it has been shown to be effective for both components of the disease. If the methotrexate is not tolerated, then I switch to either sulphasalazine (unless there is sulpha allergy) or azathioprine. If the methotrexate is tolerated, but not completely effective, I add either an antimalarial or sulphasalazine. In patients with severe psoriasis and mild arthritis I have used PUVA which works well for the skin, and has worked well for the joints. 42 D. Gladman I reserve the use of cyclosporin A and retinoids for patients with severe psoriasis and arthritis who either refuse to take methotrexate, or are unable to tolerate it. These drugs are more toxic than the others and need to be used with caution. 43 D. Gladman References Abu-Shakra, M. and Gladman, D.D. (1994). Aetiopathogenesis of psoriatic arthritis. Rheumatology Reviews 3, 1-7. Abu-Shakra, M., Gladman, D.D., Thorne, J.C., Long, J., Gough, J., and Farewell, V.T. (1995). Longterm Methotrexate therapy in Psoriatic Arthritis: Clinical and radiologic outcome. Jounral of Rheumatology, 22, 241-45. Avila, R., Pugh, D.G., Slocumb, C.H. et al (1960). Psoriatic arthritis. A roentgenologic study. Radiology, 75, 691-702. Baek, H.J., Yoo, C.D., Shin, K.C., et al. (2000). Spondylitis is the most common pattern of psoriatic arthritis in Korea. Rheumatol Int 19, 89-94. Bhalerao, J., Bowcock, A.M. (1998). The genetics of psoriasis: a complex disorder of the skin and immune system. Hum Mol Genet. 7, 10:1537-45. Battistone, M.J., Manaster, B.J., Reda, D.J., Clegg, D.O. (1999). The prevalence of sacroilitis in psoriatic arthritis: new perspectives from a large, multicenter cohort. A Department of Veterans Affairs Cooperative Study. Skeletal Radiol 28, 196-201. Barišic-Druško, V., Dobric, I., Pašic, A, et al (1994). Frequency of psoriatic arthritis in general population and among psoriatics in department of dermatology. Acta Derm Venerol (Stockh) 74 (Suppl 186), 107-108. 44 D. Gladman Black, R.L., O'Brien, W.M., Van Scott, E.J., Auerbach, R., Eisen, A.Z., and Bunim, J.J. (1964). Methotrexate therapy in psoriatic arthritis. Double blind study on 21 patients. JAMA, 189, 743-7. Blackmore, M., Gladman D.D., Husted J., Long J., and Farewell V.T. (1995). Measuring Health Status in Psoriatic Arthritis. Jounral of Rheumatology, 22, 886-92. Bruce, I.N., Schentag, C., Gladman, D.D. (2000). Hyperuricemia in psoriatic arthritis (PsA) does not reflect the extent of skin involvement. J Clin Rheumatol 6, 6-9. Burden AD, Javed S, Hodgins M, et al (1996). Linkage to chromosome 6p and exclusion of chromosome 17q in familial psoriasis in Scotland. Br J Dermatol 135, 815-51. Buskila, D., Sukenik, S., Holcberg, G., et al. (1991) Improvement of psoriatic arthritis in a patient treated with bromocriptine for hyperprolactinemia. Jounral of Rheumatology, 18, 611-2. Buskila, D., Langevitz, P., Gladman, D.D., et al. (1992). Patients with rheumatoid arthritis are more tender than those with psoriatic arthritis. Jounral of Rheumatology, 19, 1115-9. 45 D. Gladman Cantini F, Salvarani C, Olivieri I, et al. (2001). Distal extremity swelling with pitting edema in psoriatic arthritis: a case-control study. Clin Exp Rheumatol 19, 291-296. Carrett, S. and Calin, A. (1989). Evaluation of auranofin in psoriatic arthritis: a double blind placebo controlled trial. Arthritis Rheum, 32, 158-65. Cats, A. (1990) Psoriasis and Arthritis. Cutis, 46, 323-9. Chaudhari, U., Romano, P., Mulcahy, L.D., Dooley, L.T., Baker, D.G., Gottlieb A.B. (2001). Efficacy and safety of infliximab monotherapy for plaquetype psoriasis: a randomised trial. Lancet 357, 1842-7. Clegg, D.O., Reda, D.J., Mejias E., et al: Comparison of sulfasalazine and placebo in the treatment of psoriatic arthritis. A Department of Veterans Affairs Cooperative Study. Arthritis Rheum 39, 2013-20. Cohen, M.R., Reda, D.J., Clegg, D.O. (1999). Baseline relationships between psoriasis and psoriatic arthritis: analysis of 221 patients with active psoriatic arthritis. Department of Veterans Affairs Cooperative Study Group on Seronegative Spondyloarthropathies. Jounral of Rheumatology 26:1752-56. 46 D. Gladman Combe, B., Goupille, P, Kuntz, J.L., Tebib, J., Liote, F., Bregeon, C. (1996). Sulphasalazine in psoriatic arthritis: a randomized, multicentre, placebocontrolled study. Britishish Journal of Rheumatol 35, 664-8. Costello, P., Bresnihan B, O’Farrell, C., Fitzgerald, O. (1999). Predominance of CD8+ T lymphocytes in Psoriatic Arthritis. Journal of Rheumatology 26, 1117-24. Coulton, B.L., Thomson, K., Symmons, D.P.M. and Popert, A.J. (1989). Outcome in patients hospitalised for psoriatic arthritis. Clin Rheumatol, 2, 261-65. Dowart, B.B., Gall, E.P., Schumacher, H.R. and Krauser, R.E. (1978). Chrysotherapy in psoriatic arthritis: efficacy and toxicity compared to rheumatoid arthritis. Arthritis Rheum, 21, 513-15. Duffy, C.M., Watanabe Duffy, K.N., Gladman, D.D., et al. (1992). Utilization of the Arthritis Impact Measurement Scales (AIMS) for patients with psoriatic arthritis. Jounral of Rheumatology, 19, 1727-32. Eastmond, C.J. and Wright, V. (1979). The nail dystrophy of psoriatic arthritis. Annals of Rheumatic Diseases, 38, 226-8. Eastmond, C.J. (1994). Genetics and HLA antigens. Baillières Clinical Rheumatology, 8, 263-76. 47 D. Gladman Eccles, J.T., Wright, V. (1985). The History and Epidemiologic definition of Psoriatic Arthritis as a Distinct Entity. In Psoriatic arthritis (eds, L.H. Gerber and L.R. Espinoza), pp 1-8, Grune & Stratton, Orlando Florida. Elkayam, O., Ophir, J., Yaron, M., Caspi, D. (2000). Psoriatic arthritis: interrelationships between skin and joint manifestations related to onset, course and distribution. Clin Rheumatol 19, 301-5. Ellis, C.N., Fradin, M.S., Messana, J.M., et al, (1991). Cyclosporin for-plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med, 324, 277-84. Espinoza, L.R. (1985). Psoriatic arthritis: further epidemiologic and genetic considerations. In Psoriatic arthritis (eds, L.H. Gerber and L.R. Espinoza), pp 9-32, Grune & Stratton, Orlando Florida. Espinoza, L.R., Zakraoni, L., Espinoza, C.G., et al. (1992). Psoriatic arthritis: clinical response and side effects of methotrexate therapy. Jounral of Rheumatology, 19, 872-7. Falk, E.S. and Vandbakk, Ø. (1993). Prevalence of psoriasis in a Norwegian Lapp population. Acta Derm Venereol (Stockh) 73 (suppl 000) 6-9. 48 D. Gladman Farr, M., Kitas, G.D., Waterhouse, L., Jubb, R., Felix-Davies, D. and Bacon, P.A. (1990). Sulphasalazine in psoriatic arthritis: a double-blind placebocontrolled study. Br Jounral of Rheumatology, 29, 46-9. Fournie, B., Crognier, L., Arnaud, C., et al. (1998). Proposed classification criteria of psoriatic arthritis. A preliminary study in 260 patients. Rev Rhum (Engl Ed) 66, 446-56. Fraser, S.M., Hopkins, R., Hunter, JA, et al. (1993). Sulphasalazine in the management of psoriatic arthritis. British Jounral of Rheumatology, 32, 9235. Galluzzo, E., Lischi, D.M., Taglione, E., et al. (2000). Sonographic analysis of the ankle in patients with psoriatic arthritis. Scand Jounral of Rheumatology 29:52-55. Gladman, D.D. (1992). Psoriatic arthritis: recent advances in pathogenesis and treatment. Rheum Dis Clin North Am, 18, 247-56. Gladman, D.D. (1993). Toward unravelling the mystery of psoriatic arthritis. Arthritis Rheum, 36, 881-4. Gladman, D.D. (1994) Natural history of psoriatic arthritis. Baillières Clinical Rheumatology, 8, 379-94. 49 D. Gladman Gladman, D.D. (1995) Classification criteria for psoriatic arthritis. Baillieres Clinical Rheumatology 9, 319-329. Gladman, D.D., and Brockbank, J. (2000). Psoriatic arthritis. Expert Opinion on Investigational Drugs 9, 1511-22. Gladman, D.D. and Farewell, V.T. (1995). The role of HLA antigens as indicators of disease progression in psoriatic arthritis (PSA): Multivariate relative risk model. Arthritis Rheum, 38, 845-50. Gladman, D.D., Farewell, V.T. (1999). Progression in psoriatic arthritis: Role of time varying clinical indicators. Jounral of Rheumatology 26, 2409-13. Gladman, D.D., Anhorn, K.A.B., Schachter, R.K. and Mervart, H. (1986). HLA antigens in psoriatic arthritis. Jounral of Rheumatology, 13, 586-92. Gladman, D.D., Farewell, V.T., Husted, J., Wong, K. (1998). Mortality studies in psoriatic arthritis. Results from a single centre. II. Prognostic indicators for mortality. Arthritis Rheum 41, 1103-10. Gladman, D.D., Shuckett, R., Russell, M.L., Thorne, J.C. and Schachter, R.K. (1987). Psoriatic arthritis (PSA) - an analysis of 220 patients. Quart J Med, 62, 127-41. 50 D. Gladman Gladman, D.D., Farewell, V., Buskila, D., Goodman, R., Hamilton, L., Langevitz, P. and Thorne, J.C. (1990a). Reliability of measurements of active and damaged joints in psoriatic arthritis. Jounral of Rheumatology, 17, 62-4. Gladman, D.D., Stafford-Brady, F., Chang, C.H., Lewandowski, K. and Russell, M.L. (1990b). Clinical and radiological progression in psoriatic arthritis. Jounral of Rheumatology, 17, 809-12. Gladman, D.D., Blake, R., Brubacher, B., Farewell, V.T. (1992a). Chloroquine therapy in psoriatic arthritis. Jounral of Rheumatology, 19, 1724-6. Gladman, D.D., Brubacher, B., Buskila, D., Langevitz, P, Farewell, V.T. (1992b). Psoriatic spondyloarthropathy in men and women: a clinical, radiographic, and HLA study. Clin Invest Med, 15, 371-5. Gladman, D.D., Brubacher, B., Buskila, D., Langevitz, P., Farewell, V.T. (1993) Differences in the expression of spondyloarthropathy: a comparison between ankylosing spondylitis and psoriatic arthritis. Genetic and gender effects. Clin Invest Med, 16, 1-7. Gladman, D.D., Farewell, V.T., and Nadeau, C. (1995). Clinical indicators of progression in psoriatic arthritis (PSA): multivariate relative risk model. Jounral of Rheumatology, 22, 675-9. 51 D. Gladman Goodfield M. (1994). Skin lesions in psoriasis. Baillières Clinical Rheumatology, 8, 295-316. Gottlieb, A.B. (1988). Immunologic mechanisms in psoriasis. J Am Acad Dermatol, 18, 1276-1380. Greaves, M.W. and Camp, R.D.R. (1988). Prostaglandins, leukotrienes, phospholipase, platelet activating factor, and cytokines: an integrated approach to inflammation of human skin. Arch Dermatol, 280(suppl), S33S41. Greaves, M.W., Weinstein, G.D. (1995) Treatment of psoriasis. N Engl J Med; 322: 581-8. Grilington, F.M., Skinner, M.A., Birchall, N.M., and Tan, P.L.I. (1993). + T cells from patients with psoriatic and rheumatoid arthritis respond to streptococcal antigen. Jounral of Rheumatology, 20, 983-7. Green, L., Meyers, O.L., Gordon, W. and Briggs, B. (1981). Arthritis in psoriasis. Annals of Rheumatic Diseases, 40, 366-9. Griffith, C.E.M. (1997). Therapy for psoriatic arthritis: sometimes a conflict for psoriasis. British Jounral of Rheumatology 36, 409-12. 52 D. Gladman Gupta, A.K., Matteson, E.I., Ellis, C.N., Ho, V.C., Tellner, D.C., Voorhees, J.J. and McCune, W.J. (1989b). Cyclosporin in the treatment of psoriatic arthritis. Arch Dermatol, 125, 507-10. Hanly, J.G., Russell, M.L. and Gladman, D.D. (1988). Psoriatic spondyloarthropathy: a long term prospective study. Annals of Rheumatic Diseases, 47, 386-93. Hellgren L. (1969). Association between rheumatoid arthritis and psoriasis in total populations. Acta Rheumatology Scandinavia. 15: 316-26. Helliwell, P., Marchesoni, A., Peters, M., Barker, M., and Wright, V. (1991). A reevaluation of the osteoarticular manifestations of psoriasis. British Jounral of Rheumatology, 30, 339-45. Helliwell, P.S, Hetthen, J., Sokoll, K. et al (2000). Joint symmetry in early and late rheumatoid and psoriatic arthritis: comparison with a mathematical model. Arthritis Rheumatism 43:865-71. Hicken, G.J., Kitaoka, H.B., and Valente R.M. (1994). Foot and ankle surgery in patients with psoriasis. Clinical Orthopedics and Related Research 300, 204206. Huckins, D., Felson, D.T., Holick, M. (1990). Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study. Arthritis Rheum, 33, 1723-7. 53 D. Gladman Husted, J.A., Gladman, D.D., Farewell, V.T., Cook, R.J. (2001)Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 45, 151-8. Jeannou, J., Goupille, P., Avimadje, M.A., Zerkak, D., Valat, J.P., Fouquet, B. (1999). Cervical spine involvement in psoriatic arthritis. Revue Du Rhumatisme, English Edition 66, 695-700. Jenkinson, T., Armas, J., Evison, G., et al (1994). The cervical spine in psoriatic arthritis: a clinical and radiological study. British Jounral of Rheumatology, 33, 255-9. Jones, G., Crotty, M., Brooks, P. (2000). Interventions for psoriatic arthritis (Cochrane review). Cochrane Database Syst Rev 3, CD000212. Jones, S.M., Armas, J.B, Cohen, M.G., Lovell, C.R., Evison, G., and McHugh, N.J. (1994). Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Britishish Jounral of Rheumatology, 33, 834-9. Kammer, G.M., Soter, N.A., Gibson, D.J. and Schur, P.H. (1979). Psoriatic arthritis. A clinical, immunologic and HLA study of 100 patients. Semin Arthritis Rheum, 9, 75-97. 54 D. Gladman Kane, D, Greaney, T., Bresnihan, B., Gibney, R., FitzGerald, O. (1999). Ultrasonography in the diagnosis and management of psoriatic dactylitis. Jounral of Rheumatology 26:1746-51. Klinkhoff, A.V., Gertner, E., Chalmers, A.. Gladman, D.D., Stewart, W.D., Schachter, G.D. and Schachter, R.K. (1989) Pilot study of etretinate in psoriatic arthritis. Jounral of Rheumatology, 16, 789-91. Lambert, J.B. and Wright, V. (1977). Psoriatic spondylitis: a clinical and radiological description of the spine in psoriatic arthritis. Quart J Med, 46, 411-25. Lawrence, R.C., Hochberg, M.C., Kelsey, J.L., et al (1989). Estimates of the prevalence of selected arthritis and musculoskeletal diseases in the United States. Jounral of Rheumatology, 16, 427-41. Leder RO, Mansbridge JN, Hallmayer J, Hodge SE. (1998). Familial psoriasis and HLA-B: Unambiguous support for linkage in 97 published families. Hum Hered 48, 198-211. Leczinsky, C.G. (1948). The incidence of arthropathy in a ten-year series of psoriasis cases. Acta Derm Venereol, 28, 483-87. 55 D. Gladman Lehtinen, A., Traavisainen, M., and Leirisalo-Repo, M. (1994) Sonographic analysis of enthesopathy in the lower extremities of patients with spondyloarthropathy. Clin Exp Rheumatol, 12, 143-8. Leonard, D.G., O'Duffy, J.D. and Rogers, R.S. (1978). Prospective analysis of psoriatic arthritis in patients hospitalized for psoriasis. Mayo Clin Proc, 53, 511-8. Levy, J.J., Paulus, H.E., Barnett, E.V. et al (1972). A double-blind controlled evaluation of azathioprine treatment in rheumatoid arthritis and psoriatic arthritis. Arthritis Rheum, 15, 116-7. Little, H., Harvie, J.N. and Lester, R.S. (1975). Psoriatic arthritis in severe psoriasis. Can Med Assoc J, 112, 317-9. Lomholt, G. (1963). Psoriasis: prevalence, spontaneous course and genetics. A census study on the prevalence of skin diseases in the Faroe Islands. Copenhagen: GEC CAD. Luxembourg, A., Cailla, H., Roux, H. and Roudier, J. (1987). Do viruses play an etiologic role in ankylosing spondylitis and psoriatic arthritis? Clin Immunol Immunopathol, 45, 292-5. Mader, R., Gladman, D.D., Long, J., et al. (1995) Injectable gold for the treatment of psoriatic arthritis - long term follow-up. Clin Invest Med, 18, 139-43. 56 D. Gladman Marks, J.M. (1980). Psoriasis: Utilizing the treatment options. Drugs, 19, 429-36. Marsal, S., Armadans-Gil, L., Martinez, M., Gallardo, D., Ribera, A., Lience E. (1999). Clinical, radiographic and HLA associations as markers for different patterns of psoriatic arthritis. Rheumatology (Oxford) 38, 332-7. Matthews D, Fry L, et al (1996). Evidence that a locus for familial psoriasis maps to chromosome 4q. Nat Genet 13, 231. Mease, P.J., Goffe, B.S., Metz, J., VanderStoep, A., Finck, B., Burge, D.J. (2000). Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet 356, 385-90. de Misa, R.F., Azafia, J.M., Harto, A, et al. (1994). Psoriatic arthritis: one year of treatment with extracorporeal photochemotherapy. J Amer Acad of Dermatol, 30, 1037-38. Moll, J.M., Wright, V. (1973). Familial occurrence of PsA. Annals of Rheumatic Diseases 32, 181-201. Moll, J.M. (1994) The place of psoriatic arthritis in the spondarthritides. Baillières Clinical Rheumatology, 8, 395-417. Mulherin D.M., FitzGerald O., Bresnihan B. (1993). Lymphedema of the upper limb in patients with psoriatic arthritis. Semin Arthritis Rheum, 22, 350-356. 57 D. Gladman Nair RP, Henseler T, Jenisch S et al (1997). Evidence for two psoriasis susceptibility loci (HLA and 17q) and two novel candidate regions (16q and 20p) by genome-wide scan. Hum Mol Genet 6, 1349-56. O'Neill, T. and Silman, A.J. (1994). Historical background and epidemiology. Baillières Clinical Rheumatology, 8, 245-61. Paiva, E.S., Macaluso, D.C., Edwards, A., Rosenbaum, J.T. (2000). Characterisation of uveitis in patients with psoriatic arthritis. Annals of Rheumatic Diseases 59, 67-70. Palit, J., Hill, J., Capell, H.A., et al. (1990). A multicentre double-blind comparison of auranofin, intramuscular gold thiomalate and placebo in patients with psoriatic arthritis. British Jounral of Rheumatology, 29, 2803. Panayi, G. (1994). Immunology of psoriasis and psoriatic arthritis. Baillières Clinical Rheumatology, 8, 419-27. Perlman, S.G., Gerber, L.H., Roberts, M., et al. (1979). Photochemotherapy and psoriatic arthritis. A prospective study. Annals of Internal Medicine, 91, 717-22. 58 D. Gladman Peterson, A.W. and , J.P. (1992). Facia lata interpositional arthroplasty in the treatment of temporomandibular joint ankylosis caused by psoriatic arthritis. International Journal of Oral Maxillofactory Surgery, 21, 137-9. Prinz, J.C. (2001) Psoriasis vulgaris – a sterile antibacterial skin reaction mediated by cross-reactive T cells? An immunological view of the pathophysiology of psoriasis. Clinical and Experimental Dermatology 26, 326-32. Punzi, I., Pianon, M., Bertazzolo, N., et al. (1998). Clinical, laboratory and immunogenetic aspects of post-traumatic psoriatic arthritis: a study of 25 patients. Clin Exp Rheumatol 16, 277-281. Rahman, P., Gladman, D.D., Zhou, Y., Cook, R.J. (1998). The use of sulfasalazine in psoriatic arthritis: a clinic experience. Journal of Rheumatol 25,.1957-61. Rahman, P., Nguyen, E., Cheung, C., Schentag, C,T,, Gladman, D.D. (2001). Comparison of radiological severity in psoriatic arthritis and rheumatoid arthritis. Jounral of Rheumatology 28, 1041-44. Resnick, D. and Niwayama, G. (1981). Psoriatic arthritis. In Diagnosis of Bone and Joint Disorders pp 1103-29. WB Saunders Co, Philadelphia. Ritchlin, C., Haas-Smith, S.A., Hicks, D., et al. (1998). Patterns of cytokine production in psoriatic synovium. Journal of Rheumatol, 25, 1544-52. 59 D. Gladman Roberts, M.E.T., Wright, V., Hill, A.G.S. and Mehra, A.C. (1976). Psoriatic arthritis: A follow-up study. Annals of Rheumatic Diseases, 35, 206-19. Roux, H., Schiano, A., Maestracci, D. and Serratrice, G. (1979). Notre experience du traitement du rheumatisme psoriasique par la D-penicillamine. Revue du Rheumatisme, 46, 631-3. Sakkas, L.I., Loqueman, N., Bird, H., Vaughan, R.W., Welsh, K.I. and Panayi, G.S. (1990). HLA class II and T cell receptor gene polymorphisms in psoriatic arthritis and psoriasis. Jounral of Rheumatology, 17, 1487-90. Sakkas, L.I., Jachesoni, A., Kerr, L.A., et al (1991). Immunoglobulin heavy chain gene polymorphism in Italian patients with psoriasis and psoriatic arthritis. Br Jounral of Rheumatology 30, 449-50. Salvarani, C., Macchioni, P., Cremonesi, T., et al (1992a). The cervical spine in patients with psoriatic arthritis: a clinical, radiological and immunogenetic study. Annals of Rheumatic Diseases, 51, 73-7. Salvarani, C., Macchioni, P., Boiardi, L., et al. (1992b). Low dose cyclosporin A: relation between soluble interleukin-2 receptors and response to therapy. Jounral of Rheumatology, 19, 74-9. 60 D. Gladman Salvarani, C., Socco G.L., Macchioni, P., et al. (1995). Prevalence of psoriatic arthritis in Italian patients with psoriasis. Jounral of Rheumatology, 22, 1499-1503. Salvarani,C., Cantini, F., Olivieri, I., et al. (1997). Isolated peripheral enthesitis and/or dactylitis: a subset of psoriatic arthritis. Jounral of Rheumatology 24, 1106-10. Salvarani, C., Cantini, F., Olivieri, I., et al (1999). Distal extremity swelling with pitting edema in psoriatic arthritis: evidence of 2 pathological mechanisms. Jounral of Rheumatology 26, 1831-4. Samuelsson L, Dnlund F, Torinsson A, et al. (1999). A genome-wide search for genes predisposing to familial psoriasis by using a stratification approach. Hum Genet 105, 523-9. Scarpa R. (2000). Discovertebral erosions and destruction in psoriatic arthritis. Jounral of Rheumatology 27,975-8. Scarpa, R., Oriente, P., Pulino, A., Torella, M., Vignone, L., Riccio, A. and Biondi Oriente, C. (1984). Psoriatic arthritis in psoriatic patients. British Jounral of Rheumatology, 23, 246-50. 61 D. Gladman Scarpa, R., Oriente, P., Pucino, A., Vignone, L., Cosentini, E., Minerva, A. and Biondi-Oriente, C. (1988). The clinical spectrum of psoriatic spondylitis. British Jounral of Rheumatology, 27, 123-37. Scarpa, R., Del Puente, A., di Girolamo, C, et al (1992). Interplay between environmental factors, articular involvement, and HLA B27 in patients with psoriatic arthritis. Annals of Rheumatic Diseases 51, 78-79. Shbeeb, M., Uramoto, K.M., Gibson, L.E., O'Fallon, W.M., Gabriel, S.E. (2000) The epidemiology of psoriatic arthritis in Olmsted County, Minnesota, USA, 1982-1991. Jounral of Rheumatology 27:1247-50. Soyland, E., Funk, J., Rajka, G.,et al. (1993). Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med 328, 1812-6. Smith D.L., White C.R. Jr. (1994). Pyoderma gangrenosum in association with psoriatic arthritis. Arthritis Rheum, 37:1258-60. Spadarao, A., Riccieri, V., Sili-Scavalli. A., et al. (1995). Comparison of cyclosporin A and Methotrexate in the treatment of psoriatic arthritis: a one-year prospective study. Clin Exp Rheumatol 13, 589-93. 62 D. Gladman Stern, R.S. (1985). The epidemiology of joint complaints in patients with psoriasis. Journal of Rheumatol, 12, 315-20. Taglione, E., Vatteroni, M.I., Martini, F., et al. (1999). Hepatitis C virus infection: prevalence in psoriasis and psoriatic arthritis. Journal of Rheumatology 26, 370-2. Tassiulas, I., Duncan, S.R., Centola, M., et al. (1999). Clonal characteristics of T cell infiltrates in skin and synovium of patients with psoriatic arthritis. Human Immunology 60, 479-91. Thomssen, H., Hoffmann, B., Schank, M., Elewaut, D., Meyer zum Buschenfelde, K.H., Marker-Hermann, E. (2000). There is no disease-specific role for streptococci-responsive synovial T lymphocytes in the pathogenesis of psoriatic arthritis. Med Microbiol Immunol (Berl) 188, 203-207. Tomfohrdre, J., Silverman, A., Barnes, R., et al. (1994). Gene for familial psoriasis susceptibility mapped to the distal end of human chromosome 17q. Science 264, 1141-5. Torre-Alonso, J.C., Rodriguez-Perez, A., Arribas-Castrillo, J.M., et al (1991). Psoriatic arthritis (PA): a clinical, immunological and radiological study of 180 patients. British Jounral of Rheumatology, 30, 245-50. 63 D. Gladman Trembath RC, Clough RL, Rosbotham JL, et al. (1997). Identification of a major susceptibility locus on chromosome 6p an evidence for further disease loci revealed by two stage genome-wide search in psoriasis. Hum Mol Genet 6, 813-20. Vasey, F.B. (1985). Etiology and pathogenesis of psoriatic arthritis. In Psoriatic arthritis (eds. L.H. Gerber and L.R. Espinoza), pp 45-57, Grune & Stratton, Orlando, Florida. Vasey, F.B., Seleznick, M.J., Fenske, N.A. and Espinoza, L.R. (1989). New signposts on the road to understanding psoriatic arthritis. Jounral of Rheumatology, 16, 1405-7. Veal CD, Clogh RL, Barber RC, et al. (2001). Identification of a novel psoriasis susceptibility locus at 1p and evidence of epistasis between PSORI1 and candidate loci. J Med Genet 38, 7-13. Veale, D., Barrell, M., Fitzgerald, O. (1993a). Mecahnisms of joint sparing in a patient with unilateral psoriatic arthritis and a long-standing hemiplegia. British Journal of Rheumatology 32, 413-6. Veale, D., Yanni, G., Rogers, S., Barnes L, Bresnihan, B., and Fitzgerald, O. (1993b). Reduced synovial membrane macrophage numbers, ELAM-1 64 D. Gladman expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum, 36, 893-900. Veale, D.J., Barnes, L., Rogers, S., and FitzGerald, O. (1994a). Immunohistochemical markers for arthritis in psoriasis. Annals of Rheumatic Diseases, 53, 450-4. Veale, D., Rogers, S. and Fitzgerald, O. (1994b). Classification of clinical subsets in psoriatic arthritis. British Jounral of Rheumatology, 33, 133-8. Veale, D.J., Torley, H.I., Richards, I. M., et al (1994c). A double-blind placebo controlled trial of efamol marine on skin and joint symptoms of psoriatic arthritis. British Jounral of Rheumatology, 33, 954-8. Voorhees, J.J. (1983). Leukotrienes and other lipoxygenase products in the pathogenesis and therapy of psoriasis and other dermatoses. Arch Dermatol, 119, 541-7. Willkens, R.F., Williams, H.J., Ward, J.R., et al. (1984). Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum, 27, 376-81. Wong, K., Gladman, D.D., Husted, J., Long, J., Farewell, V.T. (1997). Mortality studies in psoriatic arthritis. Results from a single centre. I. Risk and Causes of Death. Arthritis Rheum 40, 1868-72. 65 D. Gladman Wright, V. (1956). Psoriasis and Arthritis. Annals of Rheumatic Diseases, 15, 34853. Wright, V. and Moll, J.M.H. (1971). Psoriatic arthritis. Bull Rheum Dis, 21, 62732. Wright, V. and Moll, J.M.H. (eds.)(1976). Psoriatic arthritis. In Seronegative polyarthritis pp 169-223, North Holland Publishing Co., Amsterdam. Wright, V., Roberts, M.C. and Hill, A.G.S. (1979). Dermatological manifestations in psoriatic arthritis: a follow-up study. Acta Dermato Venereol, 59, 235-40. Wrone-Smith, T., Nickoloff, B.J. (1996). Dermal injection of immunocytes induces psoriasis. J Clin Invest 98, 1878-87. Zacharias, H. and Zacharias, E. (1987). Methotrexate treatment of psoriatic arthritis. Acta Dermatol Venereol, 67, 270-3. Zanelli, M.D. and Wilde, J.S. (1992) Joint complaints in psoriasis patients. International Journal of Dermatology 31: 488-491. Zangger, P., Gladman, D.D., Bogoch, E.R. (1998). Musculoskeletal surgery in psoriatic arthritis. Jounral of Rheumatology 25, 725-9. Zangger, P., Esufali, Z.H., Gladman, D.D., Bogoch, E.R. (2000) Type and Outcome of Reconstructive Surgery for Different Patterns of PsA. Jounral of Rheumatology 27, 967-74. 66 D. Gladman 67 D. Gladman Legends for Figures Figure 1: Distal arthritis, involving the distal interphalangeal (DIP) joints, with erosions and joint space narrowing. Figure 2: An asymmetric oligoarthritis involving the third PIP joint on the right. Note the psoriatic lesions in the periungual areas of the left 4th and 5th fingers. Figure 3: Arthritis mutilans, which is a deforming, destructive and disabling form of arthritis, showing the inability to fully use the hands. Figure 4: Telescoping if the third DIP joint, seen in patients with psoriatic arthritis, which may be part of arthritis mutilans. Figure 5: Thoraco-lumbar spine in a patient with psoriatic spondyloarthropathy demonstrating syndesmophytes. Figure 6: Bilateral sacroiliitis in a patient with psoriatic spondyloarthropathy. Figure 7: Dactylitis, which presents as swelling of a whole digit, with inflammation involving DIP, PIP, and occasionally the MCP joints, involving the thumb and third finger. Note the psoriatic skin and nail lesions. 68 Figure 8: D. Gladman Spur formation at the insertion of the plantar fascia, representing enthesitis. Figure 9: "whittling" (lysis) of terminal phalanges of the first and second toes bilaterally. Figure 10: "pencil-in-cup" appearance seen in its early phase in the second right PIP, the fifth right DIP, and the left fifth DIP. Full developed changes are seen in the left index DIP and the left thumb IP joints. In addition, ankylosis is seen in the right DIP joint Figure 11: Fluffy periostitis in the distal end of the tibia. 69 Table 1: D. Gladman The prevalence of psoriatic arthritis among patients with psoriasis Author (year) Centre Number of Percent patients with studied arthritis Leczinsky (1948) Sweden 534 7 Vilanova (1951) Barcelona 214 25 Little (1975) Toronto 100 32 Leonard (1978) Rochester 77 39 Green (1981) Cape Town 61 42 Scarpa (1984) Naples 180 34 Stern (1985) Boston 1285 20 Zanelli (1992) Winston-Salem 459 17 Falk (1993) Kautokeino 35 17 Barisic-Drusko (1994) Osijek region 553 10 70 Salvarani (1995) Reggio-Emilia D. Gladman 205 36 71 D. Gladman Table 2: Clinical features of PSA in large reported series Feature Roberts (1976) Kammer (1979) Scarpa (1984) Gladman (1987) Helliwell (1991) Torre-Alonse (1991) Veale (1994) Jones (1994) Trobace (1994) 168 100 62 220 50 180 100 100 58 M/F 67/101 47/53 29/33 104/116 32/18 99/81 59/41 43/57 35/33 Age of onset 36-45 33-45 40-60 37 39 39 34 37.6 42 No. of patients 53 a 16 b 54 11 14 37 54a 25a 39 19c 78 Distal (%) 17 ? 7.5 12 Back (%) 5 21 21 Mutilans (%) 5 ? Sacroiliitis(%) ? Joints before Skin(%) 16 Asymmetric Oligo-Arthritis(%) Symmetric Poly-Arthritis (%) 43 e 26 50 35 33 63 40 0 0 16 1 ? 2d 6 7 4 6 ? 2.3 16 2 4 2 4 ? ? 16 27 36 20 15 6 43 30 ? 17 ? 15 ? 18 ? ?= unspecified. a=includes patients with only distal joints involved; b=14 including symmetric oligoarthritis; c=40 including asymmetric polyarthritis; d=33 including peripheral joint + back involvement;e=4 were symmetric, 72 D. Gladman Table 3: comparison between psoriatic arthritis and rheumatoid arthritis Psoriatic Rheumatoid arthritis arthritis Female preponderance Uncommon Common DIP involvement Common Uncommon Symmetry Less common Common Common Uncommon Erythema over affected joint Back involvement Common Uncommon Enthesopathy Common Uncommon Skin lesions Common Uncommon Nail lesions Common Uncommon Rheumatoid factor Uncommon Common Osteopenia Uncommon Common Osteolysis Common Uncommon Ankylosis Common Uncommon 73 D. Gladman Therapeutic approach to the management of psoriatic arthritis Type of presentation NSAIDS 2nd line Intra-articular mild skin mild joint yes no p.r.n. mild skin moderate joint yes sulphasalazine, methotrexate, antimalarials, gold, azathioprine p.r.n. severe skin mild joint yes methotrexate, sulphasalazine, cyclosporin A, PUVA, retinoids p.r.n. severe skin severe joint yes methotrexate, sulphasalazine, azathioprine, PUVA, cyclosporine A, retimoids. p.r.n