Chapter 8 - Evaporation
advertisement
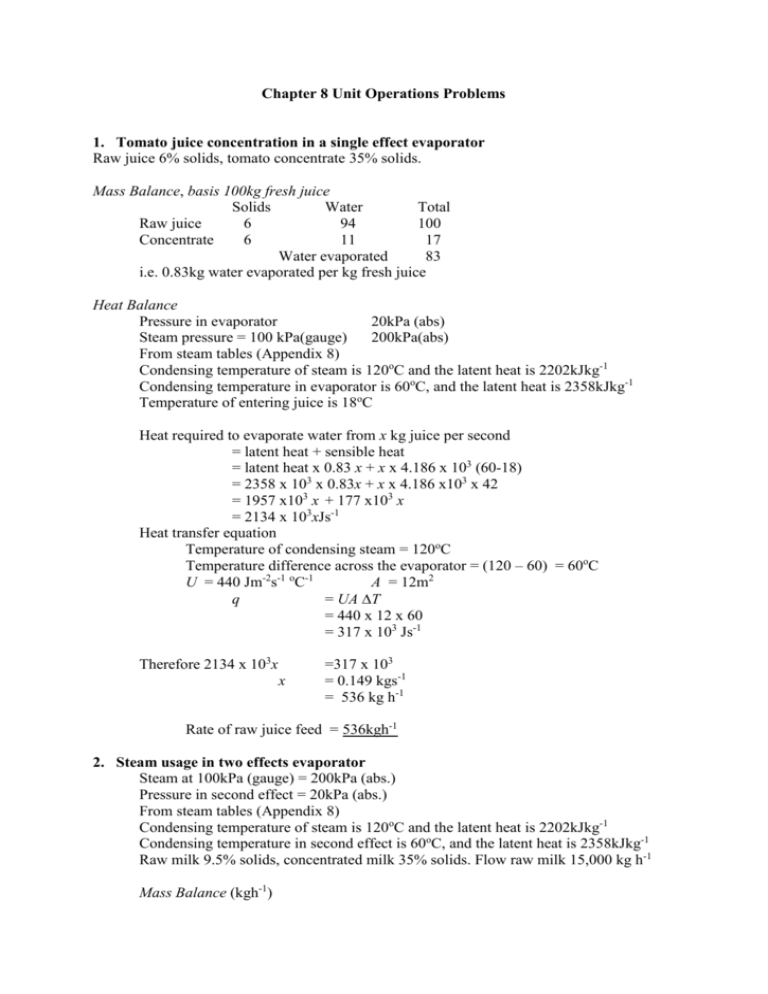
Chapter 8 Unit Operations Problems 1. Tomato juice concentration in a single effect evaporator Raw juice 6% solids, tomato concentrate 35% solids. Mass Balance, basis 100kg fresh juice Solids Water Total Raw juice 6 94 100 Concentrate 6 11 17 Water evaporated 83 i.e. 0.83kg water evaporated per kg fresh juice Heat Balance Pressure in evaporator 20kPa (abs) Steam pressure = 100 kPa(gauge) 200kPa(abs) From steam tables (Appendix 8) Condensing temperature of steam is 120oC and the latent heat is 2202kJkg-1 Condensing temperature in evaporator is 60oC, and the latent heat is 2358kJkg-1 Temperature of entering juice is 18oC Heat required to evaporate water from x kg juice per second = latent heat + sensible heat = latent heat x 0.83 x + x x 4.186 x 103 (60-18) = 2358 x 103 x 0.83x + x x 4.186 x103 x 42 = 1957 x103 x + 177 x103 x = 2134 x 103xJs-1 Heat transfer equation Temperature of condensing steam = 120oC Temperature difference across the evaporator = (120 – 60) = 60oC U = 440 Jm-2s-1 oC-1 A = 12m2 q = UA T = 440 x 12 x 60 = 317 x 103 Js-1 Therefore 2134 x 103x x =317 x 103 = 0.149 kgs-1 = 536 kg h-1 Rate of raw juice feed = 536kgh-1 2. Steam usage in two effects evaporator Steam at 100kPa (gauge) = 200kPa (abs.) Pressure in second effect = 20kPa (abs.) From steam tables (Appendix 8) Condensing temperature of steam is 120oC and the latent heat is 2202kJkg-1 Condensing temperature in second effect is 60oC, and the latent heat is 2358kJkg-1 Raw milk 9.5% solids, concentrated milk 35% solids. Flow raw milk 15,000 kg h-1 Mass Balance (kgh-1) Raw milk Concentrated milk Solids 1425 1425 Heat Balance (Js-1) q1 U1 A1 T1 Liquids 13,375 2,646 Evaporated water Total 15,000 4,071 10,929 = q2 = U2 A2 T2 U1 = 600 Jm-2s-1 oC-1 U2 = 450 Jm-2s-1 oC-1 A1 = A2 T1 + T2 = (120 –60) oC = 60 oC T2 = 60 - T1 600 A T1 600 T1 1050 T1 T1 T2 = 450 A (60 - T1) = 450 (60 - T1) = 27 x 103 – 450 T1 =27 x 103 = 25.7 26o C = 60 – 26 = 34oC (a) Evaporating temperatures: In first effect: (120 –26) = 94oC, latent heat = 2247kJkg-1 In second effect (94 – 34) = 60oC, latent heat = 2358kJkg-1 (b) Steam requirement ws = steam condensed per hour in effect 1 w1 = water evaporated per hour in effect 1 w2 = water evaporated per hour in effect 2 w1 x 2247 x 103 w2 ws = w2 x 2388 x 103 = ws x 2202 x 103 = w1 x 2247 / 2388 = w1 + 2247/2202 w1 + w2 w1 + 0.94 w1 1.94 w1 w1 w2 ws = 0.94 w1 = 1.02 w1 = 10,929kgh-1 = 10,929 = 10,929 kg h-1 = 5,633 kgh-1 = 5,296 kgh-1 = 5,746 kgh-1 It required 5,746 kgh-1of steam to evaporate a total of 10,929 kgh-1 of water i.e. 0.53 kg steam/kg water (c) Heat exchange surface for first effect: U1 = 600 Jm-1s-2 oC-1 T1 = 26oC q = U1 A1 T1 (5,633 x 2247 x 103)/3600 = 600 x A1 x 26 3 3516 x 10 = 15.6 x 103 x A A1 = 225 m2 As the areas are the same, the heat transfer area in each effect is 225m2 The total area for the two effects = 450 m2 3. Plate evaporator concentrating milk 10% solids in fresh to 30% solids in concentrated milk. Flow rate 1500kgh-1 Mass balance kgh-1 Fresh milk Concentrated milk Solids 150 150 Liquid 1350 350 Evaporated water Total 1500 500 1000 (a) Number of plates Steam at 200kPa(abs.), condensing temperature 120oC, latent heat 2202 kJkg-1 Evaporating temperature 75oC, latent heat 2322 kJkg-1 Heating surface per plate is 0.44m2 U = 650 Jm-2s-1 oC-1 x = no. of plates q = U A T = 650 A (120-75) Js-1 = 650 A 45 = 29.25 x 103 A Js-1 Heat to evaporate water = (1000 x 2322 x 103)/3600 = 6.45 x 105 A = (6.45 x 105)(29.25 x 103) = 22 m2 Each plate = 0.44 m2 Number of plates = 50 (b) With a film on the plates: (1/U2) = = = = U2 = 1/U + x/k 1/650 + 0.001/0.1 0.0015 + 0.01 0.0115 87 Therefore capacity of evaporator is reduced by (87)/ 650 = 0.134 Capacity of evaporator is reduced by 13% 4. Triple effect evaporator Feed 5% solids, product 25% solids. Input 10,000kgh-1 Mass Balance kgh-1 Solids Liquid Total Feed 500 9500 10,000 Product 500 1500 2,000 Water evaporated 8,000 (a) Evaporation in each effect Steam at 200kPa (abs.), condensing temperature 120oC, latent heat 2202 kJkg-1 Pressure in last effect 55kPa(abs.), condensing temperature 83oC, latent heat 2303kJkg-1 q1 U1 A1 T1 U1 = 600 Jm-2s-1oC-1U2 A1 = q2 = U2 A2 T2 = 500 Jm-2s-1oC-1 = A2 = T1 + T2 + T3 T2 = T1 U1/U2 = q3 = U3 A3 T3 A3 U3 = = (120-83) T3 = T1 U1/U3 T1 + T1 U1/U2 + T1U1/U3 T1 + T1 600/500 + T1 600/350 T1 + 1.2 T1 + 1.71T1 3.91T1 T1 T2 = 1.2 x 9.5 T3 = 1.71 x 9.5 T1 = 9.5 T2 = 11.5 T3 Evaporating temperature in first effect = 120 – 9.5 Evaporating temperature in second effect = 110.5 – 11.5 Evaporating temperature in third effect = 99-16 = 350 Jm-2s-1oC-1 A = 37oC = 37oC = 37oC = 37oC = 37oC = 9.5oC = 11.5oC = 16oC = 16oC = 110.5oC = 99oC = 83oC Latent heat in first effect = 2229 kJkg-1 Latent heat in second effect = 2260 kJkg-1 Latent heat in third effect = 2301 kJkg-1 w1 = water evaporated in first effect per hour w2 = water evaporated in second effect per hour w3 = water evaporated in third effect per hour ws = quantity of steam condensed w1 x 2229 x 103 = w2 2260 x 103 = w3 2301 x 103 3 = ws 2202 x10 w1 + w2 + w3 = 8000kgh-1 w1 + w1 2229 /2260 + w1 2229 / 2301 = 8000 w1 + 0.986w1 + 0.969w1 2.955 w1 w1 w2 w3 = 8000 = 8000 = 2707 kgh-1 = 2669 kgh-1 = 2623 kgh-1 Evaporation in each effect: 1st Effect 2707kgh-1, 2nd Effect 2669 kgh-1, 3rd Effect 2623 kgh-1 (b) Input of steam ws = 2707 x 2229/2202 = 2740 kgh-1 Quantity of steam per kg water = 2740/8000 = 0.343kgkg-1 5. Boiling Point Elevations Evaporating temperature in first effect Evaporating temperature in second effect Evaporating temperature in third effect = 110.5 + 0.60 = 99 + 1.50 = 83 + 4 = 111.1oC = 100.5oC = 87oC Latent heat in first effect = 2227 kJkg-1 Latent heat in second effect = 2256 kJkg-1 Latent heat in third effect = 2291 kJkg-1 w1 = water evaporated in first effect per hour w2 = water evaporated in second effect per hour w3 = water evaporated in third effect per hour ws = quantity of steam condensed w1 x 2227 x 103 = w2 2256 x 103 = w3 2291 x 103 = ws 2202 x103 w1 + w2 + w3 = 8000kgh-1 w1 + w1 2227 /2256 + w1 2227 / 2291 = 8000 w1 + 0.987w1 + 0.972w1 ws 2.959 w1 w1 w2 w3 = 2704 x 2227/2202 = 8000kgh-1 = 8000 = 2704 = 2669 = 2628 = 2735 Evaporation in each effect: 1st Effect 2704kgh-1, 2nd Effect 2669 kgh-1, 3rd Effect 2628 kgh-1 Quantity of steam per kg water = 2735/8000 = 0.342kgkg-1 No change in input steam required. 6. (a)Cooling in a jet condenser Temperature cooling water Temperature of hot vapour Mass flow Fresh milk Milk concentrate = 12oC Max. temperature exit water = 25 oC = 70oC Latent heat = 2334kJkg-1 -1 = 4000kgh = 9% solids = 30% solids Mass balance kgh-1 Fresh milk Concentrated milk (a) Jet condenser Heat Balance Heat removed from condensate Solids 360 360 Liquid 3640 840 Evaporated water Total 4000 1200 2800 = 2334 x 103 + (70 –25) x 4.186 x 103 = 2334 x 103 + 188 x 103 = 2522 x 103 Jkg-1 Heat taken in by cooling water = (25-12) x 4.186x103 = 54.4 x x103 Jkg-1 Quantity of heat removed from condensate = 2800 x 2522 x 103 Jh-1 = 7062 x 106 J h-1 Quantity of cooling water needed = 7062 x 106 / 54.4 x x103 = 130 x 103kgh-1 (b) Cooling in a surface condenser U = 2200 Jm-2s-1 oC-1 Mean temperature difference T = (70 – 12)/2 + (70 –25)/2 = 29 + 22.5 = 51.5oC Quantity of heat removed = 7062 x 106 Jh-1 = UA T = 2200 x A x 51.5 x 3600 A = 7062 x 106 /(2200 x 51.5 x 3600) = 17.3m2 Necessary heat transfer area is 17.3m2 Quantity of water needed q = wt. x spec.heat x T 6 7062 x 10 = w x 4.18 x 103 x ( 25 –12) w = 130 x 103 kgh-1 The water needed per hour is 130 x 103 kg Thi is the same as in the jest condenser, but in practice the jet condenser does not have 100% efficiency in use of water, and water used would be greater. 7. Mechanical recompression In the evaporator: Total water evaporated Vapours recompressed Energy used per kg vapour Steam = = = = Temperature of vapours = Total steam w = = = = = = = = = = = = = Heat available in steam Heat in returned vapour Energy used in compressor Heat energy available Steam energy saved 2800 kgh-1 1400kgh-1 160 kJkg-1 100 kPA (abs) Latent heat 2258 kJkg-1 Temp 99.6oC 70oC Latent Heat 2334 kJkg-1 (2334 x 2800) / 2258 2894kgh-1 2894 x 2258 x103 6535 x 106J 1400 x 2334 x103 3268 x 106J 160 x 103 x 1400J 224 x 106 J (3268 –224) x 106 3044 x 106 J (3044 x 106)/(6535 x 106) 0.466 46.6% 8. Calandria type evaporator No. of tubes = 100 Length of tube = 1 metre Diameter of tube = 5cm = 0.05m Area of tube = A Pressure in evaporator = 80kPa(abs,) Temperature = 93.5oC Latent heat 2274 kJkg-1 Specific heat of juice = 4.19kJkg-1oC-1 Pressure of steam = 100kPa(abs) Temperature = 99.6oC Latent heat 2258kJkg-1 A = DL = 3.14 x 0.05 x 1 = 0.157 m2 Total area for 100 tubes = 15.7 m2 Heat Balance Heat taken in by juice per kg = 2274 x 103 + (93.5 –18) x 4.19 x103 = 2274 x 103 + 316 x103 = 2590 x 10 3 Jkg-1 Heat transferred from steam jacket q = = = UA T 440 x 15.7 x (93.5 - 18) 5.216 x105Js-1 If Ju = wt of water evaporated from juice per hour 2590 x 103 Ju Ju = = 5.216 x105 x 3600 725kgh-1 Rate of evaporation is 725 kgh-1








