Oleic Acid Lab
advertisement

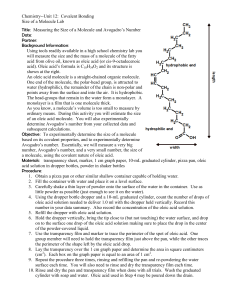
DRAFT DOCUMENT LAB 8 SIZE OF AN OLEIC ACID MOLECULE Molecules are so small that most cannot be seen in a microscope nor be measured directly even with the most sophisticated scientific equipment. However, if some physical characteristics of a certain molecule are known or can be measured, then the size of a molecule may be determined by indirect calculations. In this experiment, you will calculate the length of a molecule of oleic acid. This is a type of organic compound called a fatty acid with the formula C17H33COOH. When a drop of oleic acid is placed in water, it spreads out over the surface in a thin film. If there is sufficient room, the layer spreads until it is only one molecule thick. The –COOH part of the molecule mixes with water. The remainder of the molecule does not mix with the water but sits on top as shown in Figure 1. By measuring the thickness of the film, you will have an estimate of the length of the oleic acid molecule. OBJECTIVES When you have completed this activity, you should be able to: 1. Calculate the length of a molecule of oleic acid. 2. Express numbers in scientific notation. in order to manipulate values in algebraic equations and judge whether a solution is reasonable. 54 DRAFT DOCUMENT LAB 8 MATERIALS large shallow tray capillary pipet meter stick graduated cylinder, 10 ml lycopodium powder 0.5% oleic acid-ethanol solution ethanol (C2H5OH) PROCEDURE 1. Add water to a depth of about 1 cm in a clean tray. Make certain that the tray is level. 2. Cover the entire surface of the water with a very thin layer of lycopodium powder. 3. Add one drop of ethanol to the tray. Record your observations in Data Table 1. 4. Hold a pipet vertically over the center of the tray and close to the surface. Carefully add one drop of the 0.5% oleic acid-ethanol solution to the surface of the water. Record your observations in Data Table 1. Allow the surface to stabilize. Treat the resulting area of oleic acid as a circle. 5. Measure the diameter of the film at five different places and record in Data Table 2. 6. Determine the volume of one drop of solution by counting the number of drops of the oleic acid-ethanol solution needed to fill the 10 mL graduated cylinder to the 1.0 mL mark. Check by counting the additional number of drops to raise the volume to the 2.0 mL mark. Record this number in Data Table 1. 7. Clean the tray by washing it with hot, soapy water and rinse several times with clean water. Repeat Procedures 1, 2, 4, and 5 for a second trial. DATA Procedure Number 3 4 6 DATA TABLE 1 Observation 55 DRAFT DOCUMENT Trial #1 #2 LAB 8 1 DATA TABLE 2 Diameter Oleic Acid Film (cm) 2 3 4 5 RESULTS TABLE Trial 1 Average diameter of film Area of film Volume of 1 drop of solution Volume of oleic acid in 1 drop Length of oleic acid molecule Length of oleic acid molecule % error cm cm2 mL mL cm nm % Trial 2 cm cm2 mL mL cm nm % ANALYSIS AND CONCLUSIONS 1. Compare the observations in Procedures 3 and 4. Explain the significance of the observed differences. Record the results of Analysis questions 2 through 8 in the Results Table. 2. Compute the average diameter (d) of the oleic acid film. 3. Use the following formula to calculate the area of the oleic acid film: Area = d2/4 4. Calculate the volume of each drop of solution by dividing 1.0 mL by the number of drops per milliliter as determined in Procedure 6. 56 DRAFT DOCUMENT LAB 8 5. Calculate the volume of the oleic acid in one drop of the oleic acid-ethanol solution by multiplying the concentration of oleic acid (.005) by the volume of one drop of solution. 6. Calculate the length of the oleic acid molecule using the values for its calculated volume and area. NOTE: Since Volume = Area x Thickness, and since the film is assumed to be one molecule thick, it can be stated that the thickness of the oleic acid film is also the length of the oleic acid molecule. 7. Convert your answer for the length of the oleic acid molecule from centimeters (cm) to nanometers (nm). (1 cm = 107 nm) Why is the nanometer unit a more convenient unit to use than the centimeter? 8. Calculate percent error using 2.7 nm as the accepted value for the length of the oleic acid molecule. % error = |measured value – accepted value| x 100 accepted value 9. Avogadro’s number can be estimated from knowing the molecular mass of 1 mole of oleic acid molecules. Since we know the concentration of oleic acid, the volume of it used, we can estimate the number by using the formula below: A N = 282 V 3 Calculate Avogadro’s number using your obtained data. 57 DRAFT DOCUMENT LAB 8 PRE-LAB: SIZE OF AN OLEIC ACID MOLECULE A beaker (Figure 1) is filled with marbles to the 200 mL mark. 1. Figure 1 Assuming that the space between marbles (molecules) is negligible, what is the volume occupied by the marbles? When these marbles are spread out to one marble thickness, they cover a rectangular cake pan with the dimensions shown in Figure 2. 2. Figure 2 Calculate the area occupied by the single layer of marbles in Figure 2. 3. Using the volume from Figure 1 and the area from Figure 2, calculate the height of the marble layer, h. (Figure 3) [Hint: The volume of a rectangular solid can be calculated by multiplying area x height.] Show all your work. Include units. Figure 3 4. If 40 drops of liquid have a volume of 1.0 mL, what is the volume of one drop? 5. How many mL of oleic acid are in 1.0 mL of oleic acid solution? See Procedure 4 for the concentration of the oleic acid solutions. 58