ME 84 - Heat Transfer Laboratory
advertisement

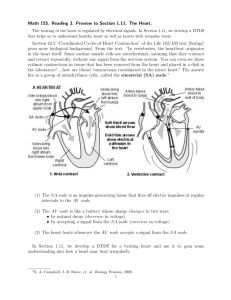
ME 315 - Heat Transfer Laboratory Experiment No. 2 TIME DEPENDENT HEAT CONDUCTION Nomenclature Cp specific heat, J/(kg K) Di inner diameter of cylinder, m Do outer diameter of cylinder, m Fo Fourier number, g gravitational constant, m/sec2 overall heat transfer coefficient, W/(m2 K) h k thermal conductivity, W/(m K) overall Nusselt number, Nu D qrad net radiative heat flux, W/m2 Pr Prandtl number, RaD Rayleigh number, t time, sec T temperature, K Th temperature of heating band, K Ti initial temperature, K T ambient temperature, K Greek Symbols thermal diffusivity, m2/s volumetric thermal expansion coefficient, 1/K surface emissivitiy, Stefan-Boltzmann constant, W/(m2 K4) kinematic viscosity, m2/s density, kg/m3 Objectives The purposes of this experiment are to measure the propagation of heat through a solid, to deduce the thermal diffusivity, and to compare the results with data available in tables. Concepts Emphasized 1. One dimensional, unsteady heat conduction. 2. Experimental techniques for measuring and analyzing transient temperatures. 3. Energy balances for control volumes in an unsteady state. 4. Modeling of boundary conditions. 2.1 Pre-Lab Section: Theoretical Analysis The purpose of this exercise is to formulate a mathematical model which can be used to calculate the temperatures in a thin walled metal cylinder, heated at one end, as functions of time and position. The model calculations can then be used to compare with those actually measured. 1. To analyze the heat flow through the solid, consider the experimental apparatus shown in Fig. 1. The heating unit, which is simply a resistor, is confined within a metal band for safety purposes. As the electrical power is turned on, electrical energy is converted into heat which is then conducted through the metal band into the hollow cylinder. Although the contact between the metal band and the hollow cylinder is quite good, it is not perfect, i.e., the effect of contact resistance could be significant. To reduce heat losses by convection to the surrounding air, the cylinder is enclosed within a transparent enclosure. T ransparent Enclosure Heater T hermocouples Metal Tube Signal Conditioning Card 70 V 1 A PC Power Supply Data Acquisition System Figure 1. Schematic diagram of overall experimental setup for measurements of timedependent heat conduction in a hollow metal cylinder. 2. Due to the effects of contact resistance, it is necessary to separately analyze the temperature history of the heating unit and the temperature history of the hollow cylinder. To perform such analysis, you should specify one control volume for the heating element and another for the hollow cylinder utilizing the provided sketches in Figs. 2 and 3. 3.81cm Heating Band Specifications mh = 0.077kg Cp,h=402.6 J/(kg K) r Node 0 r=0 x Dhi x=0 Figure 2. Cross-section of the heating band. 2.2 Heating Band Dimensions Dho=3.14 cm Dhi=2.54 cm Thickness=0.3 cm Lh=3.81 cm 3. Consider the sketch of the heating unit shown in Fig. 2. Specify the control volume, including losses/gains of energy. Complete the list of assumptions that are required for performing the energy balance: (a) the heater temperature does not vary in either the circumferential or the axial direction; (b) (c) (d) (e) 4. Perform the energy balance for the control volume treating the heater as Node 0. Your equation should be a simplification of Equation 5.15 in the text according to the above assumptions. 5. The surface heat flux term qs must be considered in this case as conduction heat transfer from the heater into the metal cylinder. To simplify calculating qs , we will make the approximation that the radial heat conduction in the band can be calculated as if it were plane conduction, as we will do in the x direction. This requires that the wall thickness of the band be much smaller than its diameter. This approximation will suffice for our calculations. Write qs in terms of the thermal conductivity and a radial temperature gradient and substitute it into your equation: 6. Now approximate the terms in this equation using finite differences between the Nodes 0 and 1, thus giving a finite difference equation for Node 0: 7. Consider the sketch of the hollow cylinder shown in Fig. 3. In a similar manner as in item 3 above, proceed by specifying the control volume for each of the eight nodes. Prior to performing the energy balance for each node, complete the following list of assumptions: (a) temperature is dependent on time (t) and the axial (x) direction only, (b) (c) (d) (e) 2.3 x=0 x Node 0 Metal Tube Node 1 2 3 4 5 6 7 8 Di Heater Figure 3. Cross-section of Metal Cylinder 8. Perform an energy balance according to the explicit method for each of the eight nodes of the hollow cylinder, starting with node 1. Follow the notation used in Sect. 5.9.1, of the text by Incropera and DeWitt; it may be useful for you to review this section as well. Here, the temperature is denoted Tmp , where the subscript m denotes the m-th node and the superscript p the time-step (or level). Rearrange your equations so that the Fo number appears in them. Note: you should be able to perform the energy balance without consulting the text. Node 1: Node 2: Nodes 3-7: Node 8: 2.4 Do The above nine energy balances (including node 0) should yield nine coupled equations for the nine unknown temperatures. Please have your instructor come by and check your work. If have time and experience, then you may wish to program these equations on the PC using Matlab. Also note that this finite difference problem can be solved using Excel by assigning a column to each node and writing the proper equation for each cell in each column. 9. The above system of equations has been programmed on the PC using C. The program can be opened by clicking on the desktop icon labeled Finite Diff Aluminum or Finite Diff StSteel. Once the graphical interface opens, run the computational model by clicking on the DO CALCULATION button. The program will plot the resulting temperature as a function of time for node 1 and node 8 and will automatically store the results for all the nodes in a file. Note approximately how long it takes for Node 1 to reach 70C. Exit the program by clicking on the QUIT button. Knowing that the Fourier number must be equal to or less than 0.5 for the computational model to remain stable, determine the time step (t) and the number of time steps that are necessary to simulate ten minutes of real time. The thermodynamic properties are specified in Table 1 below. Table 1 Properties of the Metal Cylinders. Size and Properties T-6061 Aluminum T-304 Stainless Steel Inner Diameter 0.0210 m 0.0238 m Outer Diameter 0.0254 m 0.0254 m Thermal Conductivity 177 W/(m K) 14.9 W/(m K) Thermal Diffusivity 73 10-6 m2/s 3.95 10-6 m2/s Density 2770 kg/m3 7900 kg/m3 Specific Heat 875 J/(kg K) 477 J/(kg K) 10. The results from these finite difference calculations are stored in the files C:\Temp\AluminumFD.txt and C:\Temp\StSteelFD.txt. Using Excel, import the data from the calculations for the aluminum and stainless steel cylinders. Plot the temperature as a function of time for nodes 1-8. Your graphs should resemble the one shown in Fig. 3 below. Calculated Aluminum Tube Temperatures 350 Temperature (K) Node 1 340 Node 2 330 320 310 300 290 0 20 40 60 80 100 Time (sec) Figure 3. Typical Finite Difference Results for the Aluminum Cylinder. 2.5 DAQView Setup Parameters Thermocouple Type: Units: Number of Thermocouples: Start Condition: Stop Condition: Scan Rate: Averaging: Suggested Monitoring Method: T °C 8 ( +CJC ) Manual Start Manual Stop 0.4 scan/sec (1 scan/2.5 secs) Enabled: 100 Digital Meters/Chart Experimental Procedures 1. Obtain the equipment needed for the experiment. This includes the power supply unit, stainless steel and aluminum heat conduction system, and thermocouple attachment unit. 2. Complete the connections for the stainless steel cylinder system as shown in Fig. 1. The thermocouple wires snap very easily. BE CAREFUL while handling them. DO NOT turn on the power supply. 3. Configure the data acquisition software. 4. Finally, click on the Digital Meters… icon. Using the drop down menu, select 8 meters to be displayed. The digital meter will be used to monitor the temperature of node 1, so that the power supply can be turned off and data acquisition can be stopped once the temperature of node 1 has reached 70°C. If any thermocouples behave strangely, seek help from the instructor. 5. With the heat conduction system unplugged from the power supply, turn on the power supply and adjust the voltage to 50 V for the steel cylinder or 70 V for the aluminum cylinder. Turn off the power supply and plug in the heat conduction system. 6. Turn on the power supply to the steel cylinder and make any final adjustmenst so that the voltage is set to about 50 V (if you started with the aluminum cylinder, then use 70 V). Do not rely on the dial setting; consider the meter reading as being accurate. Simultaneously, start the data acquisition. Also, click the Start button in the digital meters window so that you can monitor the temperature of node 1. 7. Once the temperature of node 1 has reached 70°C, turn off the power supply immediately (there is a danger of overheating) and stop the data acquisition and the digital meter. 8. Once the data acquisition has been stopped, the data will automatically be imported into Excel. Column A corresponds to the cold-junction circuit temperature (which can be deleted), while Columns B through I correspond to the temperatures of nodes 1 through 8. The rows correspond with time; knowing that the scan rate is 0.4 scans per second or 1 scan every 2.5 seconds, insert a column on the far left and enter the time. 9. Create a new spreadsheet and repeat steps 4 through 8 for aluminum cylinder with voltage set at 70V. 2.6 Data Analysis 1. Refer to Chapters 2 (The Heat Diffusion Equation) and 5 (Transient Conduction) of the text. Under assumptions of one-dimensional conduction heat flow, negligible convection and radiation, constant properties, and no internal heat generation the general heat diffusion equation can be reduced to: 2T 2 x 1 T t t x (1) This equation must hold for any interior point where there is no internal heat generation. It can be used to evaluate the thermal diffusivity, α by calculating the two derivatives: T t x 2T 2 x t (2) 2. Using approximations for the above partial derivatives, an Excel worksheet will be used to obtain values of from the experimental data. Find this worksheet on the ME 315 website and open it. 3. The Excel worksheet takes the output data from the data acquisition software and calculates the expected values of at the combinations of location and time given in Table 2. To use the worksheet, paste your thermocouple temperature readouts over the filler data given in the worksheet. Delete the excess rows of filler data and verify that each selected plot data series lines up with the appropriate data. Then, copy the coefficients of the polynomial curve fits into the charts on the right of the plots. The calculated value of will be reported above the polynomial tables. DO NOT change any data other than described here. If you believe you have accidentally adjusted a value in the worksheet, download the original file from the website and begin the calculation again. 4. Construct a table similar to Table 2 and enter the value of the thermal diffusivity determined from the experimental data for each of the four combinations of location and time for each material. For at least one of the combinations, record the coefficients of the polynomial fits to T T (t ) and T T ( x) so that you can calculate α for this case in a sample calculation. Determine an average value of the thermal diffusivity for each material from these values. Data Reduction. 1. Plot the experimental data for temperature as a function of time for TC locations 1-8 using Excel. 2. Tables 3 and 4 define the locations of the nodal points used in the finite difference calculations and the locations of the thermocouples used in the experiment. In a separate graph, plot the temperatures determined by the finite difference method and the experimentally determined temperatures as a function of time, for the finite difference nodes that are close to the TC locations. Show these comparisons for the pairs of points listed in Table 5. 2.7 Table 2. Locations and Times for Determining α from Experimental Data Material Positions Actual Locations (m) 4 0.080 6 0.140 3 0.055 4 0.076 Aluminum Stainless Steel Times (sec) 40 60 40 60 60 110 60 110 α (m2/s) 3. Calculate the thermal diffusivity for aluminum and steel from your experimental data using the previously recorded polynomial coefficients for the temperature curve fits. Present one complete sample calculation. Compare the experimentally determined average values of α with the values given in the Table 1. Calculate a percentage error. 4. The difference between the experimental and theoretical results may be significant. To estimate the role of radiation energy transfer, consider the formula for radiation given by A (T 4 T4 ) qrad If the emissivity 0.2 , estimate the rate of energy loss by radiation from the node 1 region at the beginning of the experiment and at the end of the experiment. 5. Calculate the predicted uncertainty of Fo and α for one for the cases given in Table 2. Discuss the meaning of the uncertainty and the effect this result has on the apparent accuracy of the experiment. 6. Use the following points to organize the discussion section of your report. (a) Compare the shapes of the curves obtained for aluminum and stainless steel. (b) Based on the nature of the curves, make qualitative comparisons of the properties of (c) (d) (e) (f) aluminum and steel. What can you say about materials that have high thermal diffusivities compared to those with lower diffusivities? Do your experimental curves match the theoretical ones at the selected nodal and thermocouple locations? Compare the average thermal diffusivity with the value tabulated in the text. Are the results reasonable? What could account for any discrepancy? Are other modes of heat transfer significant? Any other aspect of the experiment. 2.8 Table 3. Specification of thermocouple locations and nodal points used in finite-difference method for the ALUMINUM cylinder. Distance to Nodal points in Finite-difference Method 1.90 cm 5.60 cm 9.20 cm 12.8 cm 16.4 cm 20.0 cm 23.6 cm 27.2 cm Location Number 1 2 3 4 5 6 7 8 x= 0 Distance to Thermocouple Number Locations 4.0 cm 4.5 cm 6.0 cm 8.0 cm 10.0 cm 14.0 cm 21.0 cm 29.0 cm x 27.2 20.0 23.6 16.4 9.2 1.9 12.8 5.6 Node 0 Node 2 Node 1 TC1 Node 3 2.0 2.0 TC2 TC3 4.0 4.5 6.0 TC4 Node 4 2.0 TC5 Node 5 3.0 3.0 TC6 Node 6 4.0 Thermocouple Locations (cm) 8.0 10.0 14.0 21.0 29.0 Units are cm 2.9 Node 7 4.0 TC7 Node 8 4.0 TC8 Table 4. Specification of thermocouple locations and nodal points used in finite-difference method for the STAINLESS STEEL cylinder. Distance to Nodal points in Finite-difference Method 1.90 5.64 9.30 12.96 16.66 20.32 23.98 27.64 Location Number 1 2 3 4 5 6 7 8 x=0 Distance to Thermocouple Number Locations 4.08 cm 4.50 cm 5.51 cm 7.63 cm 9.17 cm 13.25 cm 20.57 cm 25.75 cm x 27.64 20.32 23.98 16.66 13.00 9.30 1.9 5.64 Node 0 2 Node 1 TC1 0.80 0.80 1.32 0.770.77 4 3 2.04 TC4 TC5 TC2 TC3 4.08 4.5 5.51 7.63 9.17 13.25 20.57 2.04 6 5 3.66 TC6 3.66 Thermocouple Locations (cm) 8 7 2.59 2.59 TC7 3.68 TC8 25.75 Units are cm Table 5. Nodal and Thermocouple Points for Temperature Comparisons ALUMINUM Nodal Point Thermocouple Number Number 2 3 3 5 6 7 STAINLESS STEEL Nodal Point Thermocouple Number Number 2 3 3 5 4 6 2.10 M.E. 315 - Heat Transfer Lab Evaluation Form EXPERIMENT #2 -- Time Dependent Heat Conduction This form is to be filled out by each student at the end of each lab experiment, and turned in with the lab report. The purpose is to help the instructor(s) make changes or modifications for the future. Your comments will in no way affect your grade--please be honest in your evaluation. 1. Approximately how much time did you spend on this experiment? ________hours in-class time, 2. 3. ________hours outside of class time What was your overall impression of the experiment? (You can elaborate on any of these in 3. below.) (a) Terrible! I hated it! Why? (b) Didn't like it much, needs these improvements: (c) Satisfactory-some minor changes needed: (d) I enjoyed it and learned a lot. Comments? (e) Fantastic! I loved it! Why? Please give any other specific comments below which will help us improve the experiment for next semester. 2.11