Balancing Equations
advertisement

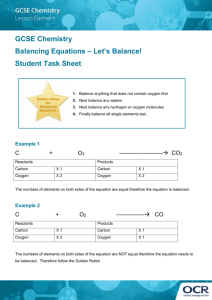
Balancing Equations ! ! A chemical equation is meant to represent an actual chemical reaction. This means that the formulas shown in the equation must accurately represent the substances in the reaction. It also means that we cannot violate any natural laws--such as creating or destroying matter. This is the reason we must balance our equations. ! ! Example #1 ! If we put a piece of zinc into some hydrochloric acid, a reaction will take place in which zinc chloride and hydrogen are produced. We could write this as a word equation: ! Zinc + Hydrochloric acid ---> Zinc chloride + Hydrogen ! The substances that we start out with are called reactants and the substances produced by the reaction are called products. The arrow separates the reactants from the products and the + signs separate the different substances within the reactants or products. ! This word equation describes the reaction, but we will generally want a to see a formula equation. All we need to do is replace each name with its appropriate formula. Remember that hydrogen is a diatomic element. ! Zn + HCl ---> ZnCl + H ! 2 2 This equation shows the correct formulas for all substances, but it still has a problem. Count the atoms and you see that we started with only one atom of hydrogen and one atom of chlorine in our reactants. However, in our products we have 2 atoms of each of these elements. This violates the law of conservation of matter. We cannot lose or gain matter in an ordinary chemical process. We need to balance this equation--make sure that there are the same number of atoms in the reactants as there are in the products. When we do this, ! WE MAY NOT CHANGE THE FORMULAS!! The formulas that we wrote are correct just the way they are. If we change them, they will represent something other than the substances present in this reaction. So what can we do? We can change the number of molecules (formula units) of each substance in the reaction. To indicate the number of molecules, we will add a coefficient in front of the appropriate formula. If there is no coefficient, it means that there is just one of that molecule involved in the reaction. ! Looking at our formula equation, we see that there is only one chlorine on the reactant side while there are two on the product side. We can't make the number of chlorine in the products any less because we need at least one molecule of ZnCl2. Therefore, we have to make the number of chlorine in the reactants greater. If we had two HCl molecules in the reactants, we would then have two chlorine atoms. Lets try this. Put a coefficient 2 in front of HCl. ! Zn + 2 HCl ---> ZnCl2 + H2 ! Now if we count our atoms again we see that there is still just one atom of zinc on each side of the equation, but now there are two chlorine on each side and there are two hydrogen on each side. When we balanced the chlorine, the hydrogen became balanced too. ! ! ! ! TIPS FOR BALANCING EQUATIONS. ! * ALWAYS BEGIN WITH CORRECT FORMULAS. * REMEMBER THE DIATOMIC ELEMENTS: H2, N2, O2, F2, Cl2, Br2, I2. * WHEN BALANCING, CHANGE ONLY THE COEFFICIENTS. * BALANCE OXYGEN LAST. * BALANCE HYDROGEN SECOND LAST. * IF YOU GET MOST OF AN EQUATION BALANCED, BUT CAN'T GET THE LAST ELEMENT TO COME OUT, TRY DOUBLING EVERYTHING YOU'VE DONE. ! ! ! Example #2 ! When we burn butane, the butane combines with oxygen from the air to produce carbon dioxide and water. We see that the reactants are butane and oxygen and the products are carbon dioxide and water. A word equation would look like this. ! Butane + Oxygen ---> Carbon dioxide + Water ! Filling in all the correct formulas we get ! C H + O ---> CO + H O ! 4 10 2 2 2 Now we need to balance the equation. Our tips say to leave oxygen for last and hydrogen for second to last, so we will begin with carbon. There are four carbon atoms in the reactants but only one atom shown in the products. In order to have four carbon atoms in the products, we would need four molecules of carbon dioxide. Lets put a coefficient 4 in front of carbon dioxide. ! CH ! 4 10 + O2 ---> 4 CO2 + H2O Now lets do the hydrogen. There are ten in the reactants but only 2 in the products. We can get ten in the products if we have 5 water molecules. ! CH ! 4 10 + O2 ---> 4 CO2 + 5H2O Now we go on to oxygen. There are two in the reactants, but 13 in the products. (Make sure you count them correctly. There are 8 atoms in the 4 carbon dioxide molecules plus 5 more in the 5 water molecules.) Here we have a little dilema. No matter how many oxygen molecules we have in the reactants, there will always be an even number of atoms. Here is where we apply that last hint. We will double everything that we've already balanced. Notice that when we double everything, we don't screw up what we've already balanced. ! 2C H ! 4 10 + O2 ---> 8 CO2 + 10 H2O The carbon and hydrogen are still balanced. Lets try the oxygen again. We still have 2 atoms in the reactants, but now we have 26 atoms in the products. We could balance this if we had 13 oxygen molecules in the reactants. ! 2C H ! 4 10 + 13 O2 ---> 8 CO2 + 10 H2O This equation was obviously more difficult to balance than the first example. However, by following the tips laid out above, we were able to balance the equation without a lot of difficulty. ! ! ! Practice Problems ! Try to write a balanced formula equation for each of the following. You can check your answers by clicking on the atom at the end of each problem. ! 1. Sodium carbonate + Calcium hydroxide ---> Sodium hydroxide + Calcium carbonate 2. Potassium chlorate ---> Potassium chloride + Oxygen 3. Lead (II) sulfide + Oxygen ---> Lead (II) oxide + Sulfur dioxide 4. Carbon disulfide + Oxygen ---> Carbon dioxide + Sulfur dioxide 5. Aluminum + Hydrochloric acid ---> Aluminum chloride + Hydrogen !