AP Chemistry: A Sample Formal Laboratory Report
advertisement
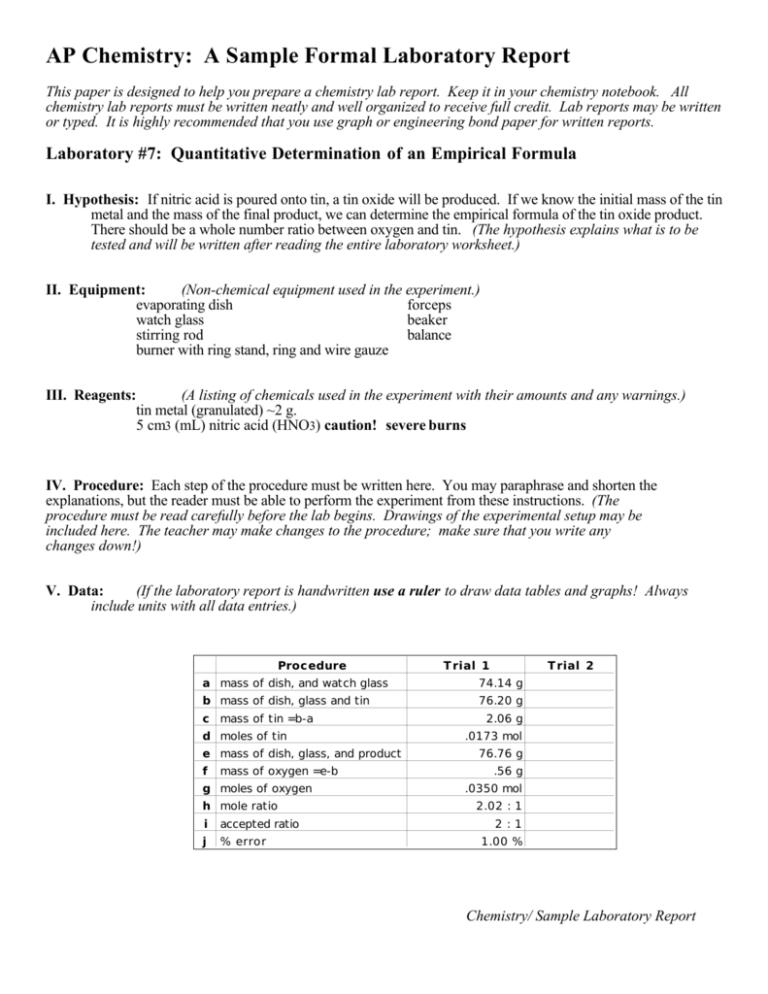
AP Chemistry: A Sample Formal Laboratory Report This paper is designed to help you prepare a chemistry lab report. Keep it in your chemistry notebook. All chemistry lab reports must be written neatly and well organized to receive full credit. Lab reports may be written or typed. It is highly recommended that you use graph or engineering bond paper for written reports. Laboratory #7: Quantitative Determination of an Empirical Formula I. Hypothesis: If nitric acid is poured onto tin, a tin oxide will be produced. If we know the initial mass of the tin metal and the mass of the final product, we can determine the empirical formula of the tin oxide product. There should be a whole number ratio between oxygen and tin. (The hypothesis explains what is to be tested and will be written after reading the entire laboratory worksheet.) II. Equipment: (Non-chemical equipment used in the experiment.) evaporating dish forceps watch glass beaker stirring rod balance burner with ring stand, ring and wire gauze III. Reagents: (A listing of chemicals used in the experiment with their amounts and any warnings.) tin metal (granulated) ~2 g. 5 cm3 (mL) nitric acid (HNO3) caution! severe burns IV. Procedure: Each step of the procedure must be written here. You may paraphrase and shorten the explanations, but the reader must be able to perform the experiment from these instructions. (The procedure must be read carefully before the lab begins. Drawings of the experimental setup may be included here. The teacher may make changes to the procedure; make sure that you write any changes down!) V. Data: (If the laboratory report is handwritten use a ruler to draw data tables and graphs! Always include units with all data entries.) Procedure Trial 1 Trial 2 a mass of dish, and watch glass 74.14 g b mass of dish, glass and tin 76.20 g c mass of tin =b-a d moles of tin e mass of dish, glass, and product f mass of oxygen =e-b g moles of oxygen h mole ratio i accepted ratio j % error 2.06 g .0173 mol 76.76 g .56 g .0350 mol 2.02 : 1 2:1 1.00 % Chemistry/ Sample Laboratory Report Laboratory #7, continued VI. Calculations: (All calculations necessary for reaching the objective of the laboratory showing all work with units and significant digits. Obvious calculations such as addition and subtraction need not be included. Moles of Sn = mass of Sn atomic mass of Sn = 2.06 g 119 g/mol = .0173 mol Sn Moles of O = mass of O atomic mass of O = 0.56 g 16.0 g/mol = .0350 mol Sn Molar ratio of oxygen to tin = mol of O mol of Sn = .0350 mol .0173 mol = 2.02 or 2 to 1 Experimental error = |experimental - theoretical| x 100 = |2.02 - 2| x 100 = 1.00 % theoretical 2 VII. Data analysis/ conclusions: (This part succinctly explains what occurred during the experiment, how the objective was reached and what the data tell us. Also, any special circumstances or problems with the experiment are stated here. In quantitative labs, an error statement with possible sources of error is necessary. This is the most important part of the carefully!) lab; write it Nitric acid is a oxidizing agent. By allowing the nitric acid to react with the granulated tin metal we were able to produce a tin-oxygen product called tin (X) oxide. By knowing the mass of the Sn reacted in the experiment and the mass of the oxygen in the product, we were able to determine the empirical formula of the product by converting these masses to moles and then dividing the greater mole quantity by the lesser. There were 2.06 g of Sn (.0173 mol) that reacted with .56 g O (.0350 mol.) The mole ratio of oxygen to tin in this experiment is approximately 2 to 1. Since tin has potential oxidation numbers of 2+ and 4+ and oxygen 2-, the product tin (IV) oxide (SnO2) is a possible product. Our actual mole ratio of 2.02 to 1 is only 1% from the whole number ratio of 2 to 1. Possible sources of error could arise from not allowing the product to dry enough, splattering of chemical during heating, and oxygen is not the only gas in the atmosphere; a small amount of tin nitride my have been produced causing a difference in the mass. If the experiment were repeated, the tin and nitric acid mixture must be heated slowly to prevent splattering and loss a product. Chemistry/ Sample Laboratory Report