The Properties of X-Rays and Atomic Spectra
advertisement

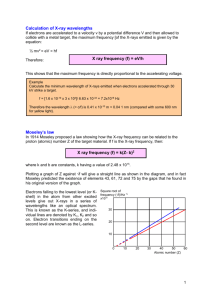
Full Experiments Spectra The Properties of X-rays and Atomic The Properties of X-Rays and Atomic Spectra Learning Outcomes (what skills you will acquire) Learn the operation of a computer controlled X-ray apparatus and goniometer; Learn the principles of X-ray generation and X-ray spectra recorded by means of Bragg diffraction; An understanding of the energy spectra as a superposition of the continuum of bremsstrahlung radiation and the lines of the characteristic X-ray radiation of the anode material; Learn the fundamentals of Bragg diffraction and the application of Bragg’s Law to crystallography; Learn about X-ray filters and absorption edges; Keeping of, and submitting, a clearly laid out set of laboratory notes. Preparatory Task Read the entire script and familiarise yourself with the learning outcomes and skills required. If you are not confident that you have so far acquired the skills you will be making use of in today’s investigation, make use of the web resources to gain further practice at http://labs.physics.dur.ac.uk/skills/general/guide.php. Specific information about this experiment can be found at http://labs.physics.dur.ac.uk/level1/projects/x-rays. Write a five to ten line summary of the aims and key physics of the experiment you will be undertaking and answer the question below in your laboratory notebook. 1) The energy of the transitions corresponding to the Mo 𝐾 , 𝐾 and 𝐿 lines are 17.479, 19.608 and 2.293 keV respectively. Calculate the wavelengths of the three lines in Ångstroms (1Å = 0.1 nm). This investigation comprises 2 related experiments: A. Investigating the energy spectrum of an X-ray tube as a function of the emission current and the high voltage. B. The Duane-Hunt relation and determination of Planck’s constant. Do not waste time, as these experiments will take the full three hours. Further background information can be found in “University Physics”, Young and Freedman , Chapter 38, pg. 1330 and Chapter 36, pgs 1250-2. Full Experiments Spectra The Properties of X-rays and Atomic Safety notes The built-in protection and screening measures reduce the local dose rate outside of the X-ray apparatus to less than 1 mSv/h, a value which is the same order of magnitude as the natural background radiation. Do not allow the Mo anode of the X-ray tube to overheat. When switching on the X-ray apparatus, check to make sure that the ventilator fan in the tube chamber is turning. The goniometer is positioned solely by electric stepper motors. Therefore do not block the target arm and sensor arm of the goniometer and do not use force to move them. Part A - Investigating the energy spectrum of an Xray tube as a function of the emission current and the high voltage A.1. What it’s about This experiment records the energy spectrum of an X-ray tube with a molybdenum (Mo) anode by means of Bragg diffraction of the X-rays from a NaCl crystal. The crystal acts as an energy filter. You will examine the X-ray energy spectra which is a superposition of the continuum of bremsstrahlung radiation and the lines of the characteristic X-ray radiation of the anode material. You will investigate how the bremsstrahlung radiation and the characteristic radiation depend on the current and the high voltage. A.2. Preparation A goniometer with an NaCl crystal and a Geiger-Müller counter tube in the Bragg configuration together comprise the spectrometer. The crystal and counter tube are pivoted with respect to the incident X-ray beam in a 𝜃 − 2𝜃 coupling (see Fig. 1). According to Bragg’s law of diffraction, the scattering angle, 𝜃, corresponds to the wavelength in the following way: 𝑛𝜆 = 2𝑑 sin 𝜃 (𝐴. 1) Figure 1: Schematic diagram of diffraction of X-rays at a monocrystal and 𝜃 − 2𝜃 coupling between counter-tube angle (2𝜃) and scattering angle (𝜃). 1 collimator, 2 monocrystal, 3 GM counter tube. where 𝑑 = 282.01 pm is lattice plane spacing of NaCl crystal, and 𝑛 is the order of diffraction. In the first order of diffraction 𝒏 = 𝟏. Thus for any given angle of incidence, there is one corresponding wavelength that gets reflected, and the crystal can be treated as an energy filter. Together with the relationships valid for electromagnetic radiation, Full Experiments Spectra The 𝑣 = Properties of X-rays and Atomic 𝑐, (𝐴. 2) 𝐸 = ℎ𝑣 (𝐴. 3) 𝜆 where is frequency, 𝑐 = 2.9979 × 10 m s-1 is the speed of light, 𝐸 is energy, and ℎ is Planck’s constant, Eq. (A.1) gives either the wavelength, or the frequency, or the energy of the incident X-rays. A.3. Ready to start Loosen the knurled screw on the sample holder in the x-ray machine and lay the NaCl crystal (Wear the plastic gloves provided to handle the crystal) flat on the target stage. Carefully raise the stage as far at it will go ensuring that the crystal is flush against the mounting lip and then tighten the knurled screw with care. !!Note: NaCl crystals are hygroscopic and fragile. Wear the plastic gloves provided to handle the crystal and avoid mechanical stresses on the crystal!! Start the software “X-ray Apparatus” and make sure that the apparatus is connected correctly. Press the ZERO key on the X-ray apparatus; this will move the sample and detector to the zero position. A.4. Measurements Varying the emission current Clear any existing measurement data using the button or the F4 key. Set the tube high voltage to 𝑈 = 35 kV. Set the emission current 𝐼 = 0.6 mA, the measuring time per angular step Δ𝑡 = 4 s and the angular step width Δ𝛽 = 0.1° Press the COUPLED key to activate 2𝜃 coupling of target and sensor and set the lower limit of the target angle to 𝛽 = 2.5° and the upper limit to 𝛽 = 12.5°. Start measuring and data transmission to the PC by pressing the SCAN key. When the measurement is finished, save your measurement under an appropriate file name by pressing the button or the F2 key. For each peak, select “Calculate Peak Centre” by right clicking anywhere in the window. Then mark the full width of the first peak by positioning the mouse on the left-hand base of the peak, and holding the mouse button down as you drag across the peak to the right-hand base of the peak. The peak will be highlighted in blue and the position and width will be reported in the bottom-left corner of the window. Record these values and repeat this process for the second peak. Copy your set of data to an Excel spread sheet (right click the mouse over the data table, and select Copy Table, then paste this data into Excel). Repeat your measurements with an emission current of 1 mA. Record the position of the alpha and beta lines in your lab book and copy the data into Excel. !!Note: One member of the team should now work on the plot (see below) in Excel. The other person should proceed to collect data for the next part of the experiment (Varying the tube high voltage)!! On your Excel plot show the y error bars on the recorded counts. Plot and print out the two intensity curves, ideally together on one sheet so that you can see any effect of varying the emission current. Full Experiments Spectra The Properties of X-rays and Atomic Hint:- Convert your count rates to a total number of counts, then evaluate the error on the number of counts. See “Estimating Counting Errors” in “Hughes and Hase” pgs 28-30”. Task 1 Show your spectra obtained at different currents. What effect does the variation of emission current, I, have on the shape of the X-ray spectrum? How does the intensity of the bremsstrahlung spectrum and the characteristic lines vary with changes of the emission current? (?) Report the positions and widths of 𝐾 and 𝐾 at the two different currents. Convert the positions to wavelengths in Ångstroms and compare these to the values calculated in the prep task. Hence identify which peak corresponds to which emission line. Bremsstrahlung radiation X-rays are created when fast-moving electrons are rapidly decelerated in matter. According to the laws of classical electrodynamics, this deceleration gives rise to electromagnetic radiation which is mainly radiated perpendicular to the direction of acceleration for energies below 50 keV, i.e. in this case perpendicular to the direction of the electrons striking the anode. For historical reasons, this X-ray component is referred to as “bremsstrahlung” after the German word for the deceleration process by which it occurs. The bremsstrahlung radiation has a continuous spectrum which extends to a certain max frequency 𝑣 or a min wavelength 𝜆 . Atomic emission lines If the energy of the electrons exceeds a critical value, characteristic X-ray radiation is generated, which appears in the spectrum as individual emission lines superimposed on the continuous bremsstrahlung spectrum. These lines are generated when high-energy electrons penetrate deep into the atomic shells of the anode material and eject electrons from the innermost orbitals. The gaps created in this process are filled by electrons from the outer orbitals. This process results in a photon (X-ray) with a characteristic energy equal to the difference in the energy of the orbitals. As the energy of an orbital is dependent on the material, the resulting X-ray radiation is characteristic of that anode material. Varying the tube high voltage Clear any existing measurement data using the button or the F4 key. Now conduct a measurement as before but use the following settings: 𝑈 = 25 kV and 𝐼 = 1.00 mA. The time and angles settings should be as before. As before make a note in your lab book of the positions of the characteristic lines. !!Note: One member of the team should now work on the plots (see below) in Excel. Ensure that it is not the same person as before. The other person should proceed to collect data for Part B experiment!! Task 2 On the same Excel graph, plot and print out your data with error bars for the two saved measurement series with 𝐼 = 1 mA (𝑈 = 25 kV 𝑎𝑛𝑑 𝑈 = 35 𝑘𝑉). Save your Excel spreadsheet. Report the positions in Ångstroms of 𝐾 and 𝐾 obtained at 25 and 35 kV. Full Experiments Spectra The Properties of X-rays and Atomic ? How does the continuum of bremsstrahlung change as the tube high voltage 𝑈 increases? How does the change in tube high voltage affect the 𝐾 and 𝐾 lines in terms of intensity and peak position? Part B - The Duane-Hunt relation and determination of Planck’s constant B.1. What it’s about In this part you will determine the minimum wavelength 𝜆 of the bremsstrahlung continuum as a function of the high voltage 𝑈 of the X-ray tube. You will then confirm the Duane-Hunt relation between the 𝜆 and the reciprocal of the high voltage, 𝑈. Finally, you will determine Planck’s constant. B.2. Preparation The bremsstrahlung continuum in the emission spectrum of an X-ray tube is characterized by 𝜆 , which becomes smaller as the tube high voltage increases (as you showed in Part A). In 1915, the American physicists William Duane and Franklin L. Hunt discovered an inverse proportionality between the minimum wavelength and the tube high voltage: 𝜆 ∝ 1/𝑈. The Duane-Hunt relationship can be sufficiently explained by examining some basic quantum mechanical considerations. The minimum wavelength 𝜆 corresponds to a maximum frequency 𝑣 (see Eq. A.2), and by Eq. A.3 to a maximum energy 𝐸 = ℎ𝑣 , (𝐵. 4) where ℎ is Planck’s constant, of the emitted X-ray quanta. The most energetic X-ray is generated when all of the kinetic energy of the incident electron is converted into radiation. 𝐸 = 𝑒 × 𝑈, (𝐵. 5) where 𝑒 = 1.6022 × 10 C is the charge on an electron. Thus, comparing Eqs. (B.4) and (B.5) and using Eq. (A.2) we get 𝑣 = 𝑒 𝑈 ; 𝜆 ℎ = ℎ𝑐 1 . (𝐵. 6) 𝑒 𝑈 Equation (B.6) corresponds to Duane and Hunt’s law. The proportionality factor, 𝐴 = ℎ𝑐 , (𝐵. 7) 𝑒 can be used to determine Planck’s constant ℎ when the quantities 𝑐 and 𝑒 are known. B.3. Measurements Conduct a measurement as in Part A with the following settings: 𝑈 = 22 kV, 𝐼 = 1.00 mA, Δ𝑡 = 30 s, Δ𝛽 = 0.1˚, 𝛽 = 5.2˚ 𝑎𝑛𝑑 𝛽 = 6.2˚. Hint: Change the x-axis from the angle to wavelength (open the “Settings” dialog with or F5 and choose “Enter NaCl” to enter the appropriate lattice plane spacing). When working in Excel use Bragg’s law to convert from angle to wavelength. Full Experiments Spectra The Properties of X-rays and Atomic Record an additional 3 measurements using the parameters from Table 1. Record all the data in the same Excel Workbook. U / kV I / mA 24 1.0 30 5.0 6.2 0.1 26 1.0 20 4.5 6.2 0.1 28 1.0 20 3.8 6.0 0.1 30 1.0 10 3.2 6.0 0.1 32 1.0 10 2.5 6.0 0.1 34 1.0 10 2.5 6.0 0.1 t/s min / grd max / grd / grd Table 1: Recommended parameters for recording the measurement series Save the measurement series. Copy each set of data to an Excel spread sheet. Save your spread sheet which should now contain 4 sets of data with an appropriate name. Task 4 To determine the minimum wavelength 𝜆 as a function of the tube high voltage 𝑈, plot counts vs. wavelength for each set of experimental parameters. Include the 𝑦error bars as you did in the previous section. For each set of data use the linest function to determine the 𝑥 intercept and its error for the points only which lie on the most linear section (or “most vertical part)” of your plot. In each case your value of 𝜆 will be the x-intercept. In Excel, plot your values of 𝜆 vs. (1/𝑈). Determine the best fit straight line through your data points. From the equation of your best fit straight line of 𝜆 =𝐴 1 𝑈 determine the slope, 𝐴 and its error (using LINEST). Evaluate Planck’s constant and its error using Eq. (B.7), and compare your value for ℎ to that found in the literature. Report your value of ℎ on the white board. The line should pass through the origin. Is your data consistent with the Duane-Hunt relation? (?) Comment on the sources of error that affect your value of Planck’s constant. What is most significant? Can you collect some additional data sets, using values in Table 1 and investigate their effect on the calculated value and error? For your extended report Think of a way of estimating the error on 𝜆 for Part B. Comment on how the error depends on your choice of the “most vertical” part. How does the error change with the high voltage? Can you use the data obtained in part A at 25 and 35 kV? Appendix:- Why do we use a single crystal of NaCl? A crystal consists of a series of repeating units of atoms or molecules made up of a lattice (a series of points in space) and a basis (the atoms themselves). The scheme of repetition (the lattice) is defined by three orthogonal vectors, a, b and c. The lattice that NaCl (as well as many other structures) is based upon is face centred cubic whose unit cell dimension, a, is 5.640 Å (1Å = 10 − 10 m) It is shown in Fig. 3a. Full Experiments Spectra The Properties of X-rays and Atomic The distance between “layers” in the NaCl crystal such as that shown in Fig. 3b is very regular and is known as the interplanar spacing, d. The separation between the planes, d, for this orientation of NaCl crystal is 2.820Å. The wavelength of X-rays is too short to be diffracted by mechanically ruled gratings except at highly grazing angles of incidence. Naturally occurring three-dimensional gratings of appropriate spacings are provided by the regular layers in crystals such as NaCl. You are using the NaCl crystal with its well defined lattice spacing and known structure as a grating, effectively as part of a spectrometer to study X-rays. However, it is important to note that this problem could be inverted. You could analyse diffraction of X-rays from a crystal of unknown material to determine its structure, both its lattice, and even its molecular chemical structure. This is the basis of X-ray crystallography, and this is how even complex biological macromolecules can be structurally solved. X-ray diffraction was the key to determining the structure of DNA in 1953 ("Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid" Nature, 171, 1953, 737-738). a) A single unit cell of NaCl b) The 3x3x3 structure of NaCl Figure 3: The crystal structure of NaCl (http://www.ilpi.com/inorganic/structures/nacl/)