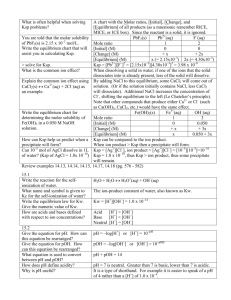
Module 1
advertisement

DANYLO HALYTSKY LVIV NATIONAL MEDICAL UNIVERSITY DEPARTMENT of GENERAL, BIOINORGANIC, PHYSICAL and COLLOIDAL CHEMISTRY V.V. Ogurtsov, O.M. Roman, O.V. Klenina MULTIPLY CHOICE QUESTIONS ON INORGANIC CHEMISTRY (Module 1. General Chemistry) For the 1st year students оf pharmaceutical faculty L’VIV – 2012 1 Chapter 1. Atomic-molecule concept. Nomenclature and classification of inorganic compounds 1.1. Compound aluminum sulfate is classified as: А. neutral salt B. acidic salt C. basic salt D. amphotheric hydroxide E. hydroxide 1.6. Compound potassium nitrate is classified as: А. acidic salt B. amphotheric hydroxide C. neutral salt D. basic salt E. hydroxide 1.2. Compound sodium hydrogencarbonate is classified as: А. acidic salt B. hydroxide C. basic salt D. amphotheric hydroxide E. neutral salt 1.7. Compound potassium hydrogencarbonate is classified as: А. hydroxide B. neutral salt C. acidic salt D. amphotheric hydroxide E. basic salt 1.3. Compound aluminium hydroxide is classified as: А. hydroxide B. basic salt C. amphotheric hydroxide D. middle salt E. acidic salt 1.8. Compound calcium phosphate is classified as: А. basic salt B. amphotheric hydroxide C. acidic salt D. hydroxide E. neutral salt 1.4. Compound lithium hydroxide is classified as: А. amphotheric hydroxide B. acidic salt C. basic salt D. neutral salt E. hydroxide 1.9. Compound strontium hydroxide is classified as: А. acidic salt B. hydroxide C. neutral salt D. amphotheric hydroxide E. basic salt 1.5. Compound beryllium hydroxide is classified as: А. amphotheric hydroxide B. acidic salt C. neutral salt D. basic salt E. hydroxide 1.10. Compound potassium sulfide is classified as: А. hydroxide B. amphotheric hydroxide C. neutral salt D. acidic salt E. basic salt 2 1.17. Give the name of an ion C2O4–: А. carbide B. hydrogencarbonate C. oxalate D. acetate E. carbonate 1.11. Compound ammonium carbonate is classified as: А. hydroxide B. neutral salt C. amphotheric hydroxide D. basic salt E. acidic salt 1.18. Give the name of an ion NO2–: А. ammonium B. nitride C. – D. nitrite E. nitrate 1.12. Compound ammonium phosphate is classified as: А. basic salt B. hydroxide C. acidics alt D. amphotheric hydroxide E. neutral salt 1.19. Give the name of an ion SO32–: А. sulfate B. sulfite C. sulfide D. thiosulphate E. sulphonate 1.13. Compound caesium hydroxide is classified as: А. neutral salt B. hydroxide C. basic salt D. amphotheric hydroxide E. acidic salt 1.20. Give the name of an ion SO42–: А. sulfite B. thiosulphate C. sulfide D. sulphonate E. sulfate 1.14. Give the name of an ion CH3COO–: А. hydrogencarbonate B. acetate C. formiate D. oxalate E. carbonate 1.21. Give the name of an ion S2–: А. sulfate B. thiosulphate C. sulfite D. sulphonate E. sulfide 1.15. Give the name of an ion CO32–: А. hydrogencarbonate B. carbonate C. oxalate D. acetate E. carbide 1.22. Give the name of an ion S2O32–: А. sulfite B. sulfate C. sulfide D. thiosulphate E. sulphonate 1.16. Give the name of an ion HCO3–: А. carbonate B. oxalate C. carbide D. acetate E. hydrogencarbonate 1.23. Give the name of an ion PO43–: А. metaphosphate B. diphosphate 3 C. phosphate D. hypophosphite E. phosphite 1.30. Ions of perchlorate and nitrate are in a pair: А. Cl–, NH4+ B. ClO–, NO2– C. ClO4–, NO3– D. Cl–, NO3– E. ClО–, NО3– 1.24. Give the name of an ion ClO–: А. chlorate B. perchlorate C. chlorite D. hypochlorite E. chloride 1.31. Ions of nitrate and cyanide are in a pair: А. NO3–, SCN– B. NO3–, CN– C. NO2–, CN– D. NO2–, CSN– E. NO3–, CN– 1.25. Give the name of an ion ClO4–: А. chlorite B. hypochlorite C. chlorate D. chloride E. perchlorate 1.32. Determine the valency of Chlorine in the following compounds KClO3 and KClO. А. VII, III B. III, II C. VII, V D. VII, I E. V, I 1.26. Give the name of an ion ClO2–: А. chlorate B. perchlorate C. chloride D. hypochlorite E. chlorite 1.27. Give the name of an ion ClO3–: А. chlorite B. perchlorate C. chlorate D. chloride E. hypochlorite 1.33. Write a molecular formula of sodium sulfide and lithium hydrogensulfate. Determine the valency of Sulfur in compounds.What is the sum of these values? А. 5 B. 6 C. 7 D. 8 E. 4 1.28. Give the name of an ion Cl–: А. perchlorate B. hypochlorite C. chlorite D. chlorate E. chloride 1.34. What is the oxidation number of a Nitrogen in the compound (NH4)2CO3 ? А. +4 B. +3 C. –3 D. –4 1.29. In which pair ions of hypochlorite and nitrite are present: А. Cl–, NO3– B. ClO4–, NO3– C. ClО–, NО3– D. ClO3–, NO2– E. ClO–, NO2– 4 E. +5 E. –4 1.35. What is the oxidation number of a Nitrogen in the compound NaNO3 ? А. –3 B. +5 C. +2 D. +3 E. +4 1.40. What is the oxidation number of a Nitrogen in the compound HNO2 ? А. +5 B. +3 C. –3 D. +2 E. +4 1.36. What is the oxidation number of a Nitrogen in the compound Ca(NО3)2 ? А. +2 B. +4 C. +3 D. +5 E. –3 1.41. What is the oxidation number of a Nitrogen in the compound NH3 ? А. +4 B. –5 C. 0 D. +3 E. –3 1.42. What is the oxidation number of a Chlorine in the compound NH4ClO4 ? А. +5 B. +1 C. –1 D. +4 E. +7 1.37. What is the oxidation number of a Nitrogen in the compound Mg(NO2)2 ? А. +3 B. +4 C. –3 D. +5 E. +2 1.43. What is the oxidation number of a Sulfur in the compound Na2SO4 ? А. –2 B. +6 C. +4 D. 0 E. +2 1.38. What is the oxidation number of a Nitrogen in the compound KNO2 ? А. +2 B. +5 C. –3 D. +3 E. +4 1.44. What is the oxidation number of a Sulfur in the compound NaHSO3 ? А. –2 B. +6 C. +4 D. +2 E. 0 1.39. What is the oxidation number of a Nitrogen in the compound NH4Cl ? А. +3 B. +4 C. –3 D. +5 1.45. What is the oxidation number of a 5 Sulfur in the compound(NH4) 2S ? А. –2 B. +4 C. +6 D. +2 E. 0 ortophosphoric acid? А. +1 B. +4 C. +3 D. +5 E. +2 1.46. What is the oxidation number of an Oxygen in the compound hydrogen peroxide H2O2 ? А. –1 B. +2 C. –2 D. +1 E. 0 1.51. What is the oxidation number of a Carbon in the compound calcium hydrogencarbonate ? А. –2 B. 0 C. +4 D. –4 E. +2 1.47. What is the oxidation number of a Bromine in the compound calcium bromide ? А. +3 B. +5 C. +7 D. –1 E. +1 1.52. What is the oxidation number of a Carbon in the compound ammonium carbonate ? А. –2 B. 0 C. +2 D. +4 E. –4 1.48. What is the oxidation number of a Phosphorus in the compound magnesium hydrogenphosphate? А. +5 B. +2 C. +4 D. +3 E. +1 1.53. What is the oxidation number of Chlorine in the compound barium chloride? А. +1 B. +5 C. +7 D. –1 E. +3 1.49. What is the oxidation number of a Phosphorus in the compound ammonium phosphate? А. +1 B. +4 C. +3 D. +2 E. +5 1.54. What is the oxidation number of Chlorine in the compound potassium chlorate? А. +1 B. +7 C. –1 D. +3 E. +5 1.50. What is the oxidation number of a Phosphorus in the compound 1.55. What is the oxidation number of Chlorine in the compound calcium 6 hypochlorite? А. +5 B. +7 C. –1 D. +3 E. +1 D. +7 E. –1 1.57. What is the oxidation number of Chlorine in the compound potassium perchlorate? А. +5 B. –1 C. +1 D. +3 E. +7 1.56. What is the oxidation number of Chlorine in the compound potassium hypochlorite? А. +3 B. +1 C. +5 Chapter 2. Structure of atoms. The Periodic law and Periodic table by D. Mendeleev 2.1. 2.2. 2.3. E. [Kr] 4d105s25p3 What is the electronic configuration of As atom? А. [He] 2s223 B. [Kr] 4d105s25p3 C. [Ar]3d104s24p3 D. [Ne] 3s23p3 E. [Xe] 4f145d106s26p3 What is the electronic configuration of Sb atom? А. [Ar]3d104s24p3 B. [Kr] 4d105s25p3 C. [He] 2s223 D. [Ne] 3s23p3 E. [Xe] 4f145d106s26p3 What is the electronic configuration of Bі atom? А. [Ar]3d104s24p3 B. [Ne] 3s23p3 C. [Xe] 4f145d106s26p3 D. [He] 2s223 7 2.4. What is the electronic configuration of As(+3) ion? А. [Ar]3d104s24p6 B. [Ar]3d94s14p3 C. [Ar]3d104s14p1 D. [Ar]3d74s24p3 E. [Ar]3d104s24p0 2.5. What is the electronic configuration of As(-3) ion? А. [Ar]3d94s14p3 B. [Ar]3d104s24p6 C. [Ar]3d104s14p1 D. [Ar]3d74s24p3 E. [Ar]3d104s24p0 2.6. What is the electronic configuration of Sb(+3) ion? А. [Kr] 4d95s05p3 B. [Kr] 4d105s25p6 C. [Kr] 4d85s15p3 D. [Kr] 4d95s25p1 E. [Kr] 4d105s25p0 2.7. 2.8. 2.9. А. [Xe] 6s26p0 B. [Xe] 6s26p2 C. [Xe] 6s16p1 D. [Xe] 6s16p0 E. [Xe] 6s26p1 What is the electronic configuration of Bi(+3) ion? А. [Xe] 4f145d106s06p2 B. [Xe] 4f115d106s26p3 C. [Xe] 4f145d76s26p3 D. [Xe] 4f145d106s26p6 E. [Xe] 4f145d106s26p0 2.13. What is the electronic configuration of Pb4+ ion: А. [Xe] 6s16p0 B. [Xe] 6s26p0 C. [Xe] 6s16p0 D. [Xe] 6s16p1 E. [Xe] 6s06p0 What is the electronic configuration of Sb(-3) ion? А. [Kr] 4d105s25p0 B. [Kr] 4d95s05p3 C. [Kr] 4d85s15p3 D. [Kr] 4d105s25p6 E. [Kr] 4d95s25p1 2.14. What is the electronic configuration of Bromine atom in potassium bromate? А. 4s24p0 B. 4s24p5 C. 4s14p6 D. 4s04p5 E. 4s24p3 What is the electronic configuration of As(+3) ion? А. [Xe] 4f145d106s26p6 B. [Xe] 4f145d76s26p3 C. [Xe] 4f115d106s26p3 D. [Xe] 4f145d106s06p2 E. [Xe] 4f145d106s26p0 2.15. What is the electronic configuration of Chlorine atom in potassium perchlorate? А. 3s03p0 B. 3s23p0 C. 3s1p6 D. 3s13p5 E. 3s23p5 2.10. What is the electronic configuration of As atom? А. [Kr] 4d105s25p3 B. [He] 2s223 C. [Xe] 4f145d106s26p3 D. [Ar]3d104s24p3 E. [Ne] 3s23p3 2.16. What is the electronic configuration of Chlorine atom in chlorous acid? А. 3s23p5 B. 3s23p0 C. 3s23p2 D. 3s13p5 E. 3s1p6 2.11. Which of the listed electronic configurations corresponds to Fe3+ion А. [Ar] 3d5 4s1 4p1 B. [Ar] 3d4 4s1 C. [Ar] 3d5 4s0 D. [Ar] 3d3 4s1 4p1 E. [Ar] 3d3 4s2 2.17. What is the electronic configuration of Bromine atom in potassium hypobromite? А. 4s24p5 2.12. What is the electronic configuration of Pb2+ ion: 8 B. C. D. E. 5s25p4 4s24p0 5s25p5 4s24p4 E. [Xe]4f145d106s0 2.23. Which of the listed electronic configurations corresponds to 29Сu atom? А. [Ar] 3d10 4s1 B. [Ar] 3d7 4s2 C. [Ar] 3d8 4s2 D. [Ar] 3d9 4s2 E. [Ar] 3d6 4s2 2.18. What is the electronic configuration of І– ion? А. 6s26p4 B. 5s25p4 C. 6s26p5 D. 5s25p5 E. 5s25p6 2.24. Which of the listed electronic configurations corresponds to Cu2+ ion ? А. [Ar]3d104s1 B. [Ar]3d84s1 C. [Ar]3d104s2 D. [Ar]3d84s2 E. [Ar]3d94s0 2.19. What is the electronic configuration of F– ion? А. 2s22p4 B. 2s22p5 C. 2s02p6 D. 2s02p5 E. 2s22p6 2.25. Which of the listed electronic configurations corresponds to 47Ag atom? А. [Kr]4d105s0 B. [Kr]4d105s1 C. [Kr]4d95s2 D. [Kr]4d85s1 E. [Kr]4d85s2 2.20. What is the electronic configuration of Br– ion? А. 4s04p5 B. 4s24p6 C. 5s25p6 D. 4s14p6 E. 4s24p5 2.21. Which of the listed electronic configurations corresponds to Cd2+ ion? А. [Kr]4d105s2 B. [Kr]4d105s0 C. [Kr]4d95s0 D. [Kr]4d105s1 E. [Kr]4d85s2 2.26. What is the electronic configuration of Ag+ ion? А. [Kr]4d105s2 B. [Kr]4d85s2 C. [Kr]4d105s0 D. [Kr]4d85s1 E. [Kr]4d95s1 2.27. Which of the listed electronic configurations corresponds to 79Au atom? А. [Xe]4f145d96s2 B. [Xe]4f145d86s2 C. [Xe]4f145d96s1 D. [Xe]4f145d106s1 E. [Xe]4f145d106s2 2.22. Which of the listed electronic configurations corresponds to Hg1+ ion? А. [Xe]4f145d106s1 B. [Xe]4f145d86s1 C. [Xe]4f145d96s2 D. [Xe]4f145d106s1 9 configuration of atom corresponds to Cu2+ ion? А. [Ar]3d84s1 B. [Ar]3d104s1 C. [Ar]3d104s2 D. [Ar]3d94s0 E. [Ar]3d84s2 2.28. What is the electronic configuration of ion Au3+? А. [Xe]4f145d96s2 B. [Xe]4f145d96s1 C. [Xe]4f145d106s0 D. [Xe]4f145d86s0 E. [Xe]4f145d86s1 2.34. What from the given electronic configuration of atom corresponds to 47Ag atom? А. [Kr]4d105s1 B. [Kr]4d85s2 C. [Kr]4d95s2 D. [Kr]4d105s0 E. [Kr]4d85s1 2.29. Which of the listed electronic configurations corresponds to 13Al atom? А. [Ne] 3s23p1 B. [Ne] 3s13p0 C. [Ne] 3s23p3 D. [Ne] 3s03p0 E. [Ne] 3s23p4 2.35. What from the given electronic configuration of atom corresponds to Ag+ ion? А. [Kr]4d85s2 B. [Kr]4d85s1 C. [Kr]4d95s1 D. [Kr]4d105s2 E. [Kr]4d105s0 2.30. Which of the listed electronic configurations corresponds to 5B atom? А. [He] 2s22p4 B. [He] 2s22p1 C. [He] 2s22p2 D. [He] 2s22p3 E. [He] 2s22p5 2.36. What from the given electronic configuration of atom corresponds to 79Au atom? А. [Xe]4f145d106s2 B. [Xe]4f145d96s2 C. [Xe]4f145d106s1 D. [Xe]4f145d86s2 E. [Xe]4f145d96s1 2.31. Which of the listed electronic configurations corresponds to Al3+ ion? А. [Ne] 3s23p4 B. [Ne] 3s23p1 C. [Ne] 3s13p0 D. [Ne] 3s23p3 E. [Ne] 3s03p0 2.37. What from the given electronic configuration of atom corresponds to Au3+ ion? А. [Xe]4f145d86s1 B. [Xe]4f145d86s0 C. [Xe]4f145d96s1 D. [Xe]4f145d106s0 E. [Xe]4f145d96s2 2.32. Which of the listed electronic configurations corresponds to the р-elements of IVA groups? А. ns2np2 B. ns2np3 C. ns2np1 D. ns2np0 E. ns1np3 2.38. Which of the listed electronic 2.33. What from the given electronic 10 configurations corresponds to Mn6+ ion? А. [Ar]3d44s1 B. [Ar]3d54s0 C. [Ar]3d54s0 D. [Ar]3d04s2 E. [Ar]3d04s1 configurations corresponds to the atom of Nitrogen? А. 1s22s22p3 B. 1s22s22p4 C. 1s22s22p2 D. 1s22s22p5 E. 1s22s22p63p1 2.39. Which of the listed electronic configurations corresponds to Mn4+ ion? А. [Ar]3d24s2 B. [Ar]3d44s1 C. [Ar]3d44s0 D. [Ar]3d24s1 E. [Ar]3d14s2 2.44. Which of the listed electronic configurations corresponds to the atom of nitrogen in a nitrogen oxide (ІІІ) N2O3? А. [He] 2s22p2 B. [He] 2s22p0 C. [He] 2s22p1 D. [He] 2s22p3 E. [He] 2s12p1 2.40. Which of the listed electronic configurations corresponds to Mn 7+ ion? А. [Ar]3d54s0 B. [Ar]3d04s0 C. [Ar]3d44s0 D. [Ar]3d04s1 E. [Ar]3d54s1 2.45. Which of the listed electronic configurations corresponds to the atom of nitrogen in a nitric acid HNO3? А. [He] 2s22p3 B. [He] 2s12p1 C. [He] 2s12p2 D. [He] 2s22p2 E. [He] 2s02p0 2.41. Which of the listed electronic configurations corresponds to Mn2+ ion? А. [Ar]3d24s1 B. [Ar]3d34s2 C. [Ar]3d54s0 D. [Ar]3d04s2 E. [Ar]3d44s1 2.46. Which of the listed electronic configurations corresponds to Cr6+ ion? А. [Ar]3d04s0 B. [Ar]3d54s0 C. [Ar]3d44s0 D. [Ar]3d54s0 E. [Ar]3d04s1 2.42. Which of the listed electronic configurations corresponds to atom of Mn? А. [Ar]3d34s2 B. [Ar]3d44s2 C. [Ar]3d44s1 D. [Ar]3d64s1 E. [Ar]3d54s2 2.47. Which of the listed electronic configurations corresponds to Cr3+ ion? А. [Ar]3d54s0 B. [Ar]3d44s1 C. [Ar]3d54s1 D. [Ar]3d34s0 E. [Ar]3d44s0 2.43. Which of the listed electronic 11 2.48. Which of the listed electronic configurations corresponds to Cr2+ ion? А. [Ar]3d44s1 B. [Ar]3d24s0 C. [Ar]3d54s0 D. [Ar]3d34s0 E. [Ar]3d44s0 2.53. The same number of energy levels have atoms of elements with atomic numbers: А. 11 and 4 B. 11 and 32 C. – D. 11 and 13 E. – 2.49. Which of the listed electronic configurations corresponds to the atom of Chromium? А. [Ar]3d64s1 B. [Ar]3d44s2 C. [Ar]3d44s1 D. [Ar]3d54s1 E. [Ar]3d54s2 2.54. The same number of energy levels have atoms of elements with atomic numbers: А. 4 and 53 B. – C. 4 and 5 D. 4 and 20 E. – 2.50. Which of the listed electronic configurations corresponds to Molybdenum atom? А. [Kr]3d64s1 B. [Kr]3d44s1 C. [Kr]3d44s2 D. [Kr]3d54s2 E. [Kr]3d54s1 2.55. The same number of energy levels have atoms of elements with atomic numbers: А. – B. 20 and 1 C. 20 and 19 D. – E. 20 and 7 2.51. Which of the listed electronic configurations corresponds to the atom of Tungsten? А. [Xe]4d45s1 B. [Xe]4d65s1 C. [Xe]4d55s2 D. [Xe]4d55s1 E. [Xe]4d45s2 2.56. The same number of energy levels have atoms of elements with atomic numbers: А. 6 and 4 B. 6 and 19 C. – D. 6 and 35 E. – 2.52. The same number of energy levels have atoms of elements with atomic numbers: А. 3 and 4 B. 3 and 40 C. – D. – E. 3 and 20 2.57. The same number of energy levels have atoms of elements with atomic numbers: А. 16 and 17 B. 16 and 3 C. 16 and 5 D. – E. – 12 2.58. The same number of energy levels have atoms of elements with atomic numbers: А. – B. – C. 12 and 32 D. 12 and 11 E. 12 and 53 2.63. How many protons contain the nucleus of an atom of element with atomic number 7? А. 3 B. 7 C. 6 D. 2 E. 4 2.59. How many protons contain the nucleus of an atom of element with atomic number 21? А. 20 B. 26 C. 12 D. 25 E. 21 2.64. How many protons contain the nucleus of an atom of element with atomic number 9? А. 4 B. 6 C. 9 D. 7 E. 2 2.60. How many protons contain the nucleus of an atom of element with atomic number 2? А. 4 B. 2 C. 6 D. 5 E. 3 2.65. How many protons contain the nucleus of an atom of element with atomic number 10? А. 13 B. 14 C. 10 D. 12 E. 5 2.61. How many protons contain the nucleus of an atom of element with atomic number 4? А. 2 B. 5 C. 6 D. 3 E. 4 2.66. How many protons contain the nucleus of an atom of element with atomic number 11? А. 12 B. 14 C. 11 D. 10 E. 13 2.62. How many protons contain the nucleus of an atom of element with atomic number 6? А. 2 B. 3 C. 4 D. 5 E. 6 2.67. How many protons do contain the kernel of atom of element with a sequence number 12? А. 12 B. 9 C. 11 D. 10 E. 8 13 2.68. How many protons contain the nucleus of an atom of element with atomic number 14? А. 12 B. 11 C. 9 D. 14 E. 10 2.73. How many protons contain the nucleus of an atom of element with atomic number 23? А. 25 B. 23 C. 26 D. 22 E. 24 2.69. How many protons contain the nucleus of an atom of element with atomic number 16? А. 17 B. 18 C. 15 D. 16 E. 14 2.74. How many protons contain the nucleus of an atom of element with atomic number 25? А. 22 B. 25 C. 26 D. 24 E. 23 2.70. How many protons contain the nucleus of an atom of element with atomic number 17? А. 15 B. 18 C. 19 D. 16 E. 17 2.75. How many protons contain the nucleus of an atom of element with atomic number 26? А. 22 B. 25 C. 26 D. 23 E. 24 2.71. How many protons contain the nucleus of an atom of element with atomic number 20? А. 16 B. 18 C. 19 D. 20 E. 17 2.76. How many protons contain the nucleus of an atom of element with atomic number 27? А. 28 B. 26 C. 29 D. 25 E. 27 2.72. How many protons contain the nucleus of an atom of element with atomic number 22? А. 21 B. 16 C. 22 D. 19 E. 20 2.77. How many protons contain the nucleus of an atom of element with atomic number 28? А. 28 B. 27 C. 30 D. 29 E. 31 14 B. 10 C. 34 D. 12 E. 7 2.78. How many protons contain the nucleus of an atom of element with atomic number 29? А. 31 B. 28 C. 32 D. 30 E. 29 2.83. What is the atomic number of element, the atom of which has 1 electrons at external energy level? А. 15 B. 20 C. 18 D. 34 E. 19 2.79. What is the atomic number of element, the atom of which has 2 electrons at external energy level? А. 11 B. 20 C. 16 D. 23 E. 19 2.84. What is the atomic number of element, the atom of which has 1 electrons at external energy level? А. 31 B. 15 C. 26 D. 38 E. 37 2.80. What is the atomic number of element, the atom of which has 2 electrons at external energy level? А. 11 B. 32 C. 12 D. 16 E. 13 2.85. What is the atomic number of element, the atom of which has 1 electrons at external energy level? А. 56 B. 34 C. 14 D. 55 E. 54 2.81. What is the atomic number of element, the atom of which has 2 electrons at external energy level? А. 6 B. 15 C. 3 D. 17 E. 4 2.86. What is the atomic number of element, the atom of which has 3 electrons at external energy level? А. 34 B. 13 C. 7 D. 12 E. 14 2.82. What is the atomic number of element, the atom of which has 1 electrons at external energy level? А. 11 15 А. 20 B. 50 C. 51 D. 2 E. 49 2.87. What is the atomic number of element, the atom of which has 3 electrons at external energy level? А. 32 B. 30 C. 31 D. 15 E. 7 2.92. What is the atomic number of element, the atom of which has 5 electrons at external energy level? А. 14 B. 15 C. 20 D. 4 E. 16 2.88. What is the atomic number of element, the atom of which has 3 electrons at external energy level? А. 48 B. 50 C. 3 D. 49 E. 11 2.93. What is the atomic number of element, the atom of which has 5 electrons at external energy level? А. 2 B. 19 C. 32 D. 33 E. 34 2.89. What is the atomic number of element, the atom of which has 4 electrons at external energy level? А. 3 B. 14 C. 20 D. 15 E. 13 2.94. What is the atomic number of element, the atom of which has 5 electrons at external energy level? А. 52 B. 3 C. 51 D. 50 E. 19 2.90. What is the atomic number of element, the atom of which has 4 electrons at external energy level? А. 16 B. 32 C. 31 D. 11 E. 33 2.95. What is the atomic number of element, the atom of which has 6 electrons at external energy level? А. 17 B. 16 C. 15 D. 3 E. 20 2.91. What is the atomic number of element, the atom of which has 4 electrons at external energy level? 16 B. 8 C. 10 D. 20 E. 11 2.96. What is the atomic number of element, the atom of which has 6 electrons at external energy level? А. 35 B. 4 C. 34 D. 19 E. 33 2.99. What is the atomic number of element, the atom of which has 7 electrons at external energy level? А. 20 B. 16 C. 19 D. 3 E. 17 2.97. What is the atomic number of element, the atom of which has 6 electrons at external energy level? А. 53 B. 11 C. 51 D. 20 E. 52 2.100. What is the atomic number of element, the atom of which has 7 electrons at external energy level? А. 20 B. 34 C. 11 D. 36 E. 35 2.98. What is the atomic number of element, the atom of which has 7electrons at external energy level? А. 9 Chapter 3. Equivavlents of substances in chemical reactions is 20 g/mole? А. 4.5 B. 12 C. 5 D. 7 E. 28 3.1. What is the equivalent mass of a metal if its oxide equivalent mass is 64.2 g/mole? А. 26.4 B. 16.0 C. 56.2 D. 13.2 E. 32.1 3.3. What is the equivalent mass of a metal if its oxide equivalent mass is 31 g/mole? А. 23 B. 5 3.2. What is the equivalent mass of a metal if its oxide equivalent mass 17 C. 12 D. 7 E. 4.5 C. 7 D. 5 E. 12 3.4. What is the equivalent mass of a metal if its oxide equivalent mass is 12.5 g/mole? А. 5 B. 8 C. 7 D. 1 E. 4.5 3.9. What is the equivalent mass of an element if its oxide equivalent mass is 14.2 g/mole? А. 12 B. 5 C. 7 D. 6.2 E. 4.5 3.5. What is the equivalent mass of a metal if its oxide equivalent mass is 51.8 g/mole? А. 26.4 B. 32.1 C. 13.2 D. 16.0 E. 43.8 3.10. What is the equivalent mass of an element if its oxide equivalent mass is 115 g/mole? А. 26.4 B. 32.1 C. 56.2 D. 16.0 E. 107 3.6. What is the equivalent mass of an element if its oxide equivalent mass is 16 g/mole? А. 4.5 B. 8 C. 12 D. 7 E. 5 3.11. What is the equivalent mass of a metal if its sulfate equivalent mass is 60.2 g/mole? А. 28 B. 12.2 C. 7 D. 4.5 E. 5 3.7. What is the equivalent mass of an element if its oxide equivalent mass is 13.3 g/mole? А. 4.5 B. 7 C. 28 D. 5.3 E. 5 3.12. What is the equivalent mass of a metal if its sulfate equivalent mass is 68 g/mole? А. 5 B. 4.5 C. 12 D. 20 E. 7 3.8. What is the equivalent mass of an element if its oxide equivalent mass is 11 g/mole? А. 4.5 B. 3 3.13. What is the equivalent mass of a metal if its sulfate equivalent mass is 57 g/mole? А. 7 B. 9 18 C. 12 D. 5 E. 4.5 C. 5 D. 23 E. 12 3.14. What is the equivalent mass of a metal if its nitrate equivalent mass is 85 g/mole? А. 4.5 B. 23 C. 7 D. 5 E. 12 3.19. What is the equivalent mass of a metal if its carbonate equivalent mass is 98.7 g/mole? А. 56.2 B. 68.7 C. 32.1 D. 16.0 E. 26.4 3.15. What is the equivalent mass of a metal if its nitrate equivalent mass is 94.7 g/mole? А. 7 B. 23 C. 12 D. 5 E. 32.7 3.20. What is the equivalent mass of a metal if its chloride equivalent mass is 58.5 g/mole? А. 4.5 B. 7 C. 12 D. 5 E. 23 3.16. What is the equivalent mass of a metal if its nitrate equivalent mass is 118.2 g/mole? А. 26.4 B. 13.2 C. 16.0 D. 32.1 E. 56.2 3.21. What is the equivalent mass of a metal if its chloride equivalent mass is 104.2 g/mole? А. 26.4 B. 68.7 C. 56.2 D. 32.1 E. 16.0 3.17. What is the equivalent mass of a metal if its carbonate equivalent mass is 50 g/mole? А. 12 B. 20 C. 30 D. 7 E. 5 3.22. What is the equivalent mass of a metal if its chloride equivalent mass is 40 g/mole? А. 1 B. 5 C. 8 D. 7 E. 4.5 3.18. What is the equivalent mass of a metal if its carbonate equivalent mass is 53 g/mole? А. 7 B. 30 3.23. What is the equivalent mass of a metal if its hydroxide equivalent mass is 37 g/mole? А. 20 B. 12 19 C. 5 D. 7 E. 30 C. 16.0 D. 26.4 E. 56.2 3.24. What is the equivalent mass of a metal if its hydroxide equivalent mass is 49.7 g/mole? А. 5 B. 23 C. 32.7 D. 12 E. 7 3.29. What is the equivalent mass of a metal hydroxide if the metal equivalent mass is 68.7 g/mole? А. 26.4 B. 32.1 C. 85.7 D. 68.7 E. 56.2 3.25. What is the equivalent mass of a metal if its hydroxide equivalent mass is 40 g/mole? А. 12 B. 7 C. 23 D. 4.5 E. 5 3.30. What is the equivalent mass of a metal hydroxide if the metal equivalent mass is 9 g/mole? А. 23 B. 7 C. 12 D. 5 E. 26 3.26. What is the equivalent mass of a metal if its hydroxide equivalent mass is 60.7 g/mole? А. 26.4 B. 32.1 C. 43.8 D. 13.2 E. 16.0 3.31. Calculate the number of equivalents of potassium hydroxide KOH which will react completely with 1 mole of sulfuric acid H2SO4. А. 0.25 B. 2 C. 4 D. 1 E. 0.5 3.27. What is the equivalent mass of a metal hydroxide if the metal equivalent mass is 56.2 g/mole? А. 73.2 B. 26.4 C. 56.2 D. 32.1 E. 68.7 3.32. Calculate the number of equivalents of sodium hydroxide NaOH which will react completely with 1 mole of sulfuric acid H2SO4. А. 1 B. 2 C. 0.5 D. 0.25 E. 4 3.28. What is the equivalent mass of a metal hydroxide if the metal equivalent mass is 39 g/mole? А. 13.2 B. 32.1 3.33. Calculate the number of 20 equivalents of calcium hydroxide Ca(OH)2 which will react completely with 1 mole of sulfuric acid H2SO4. А. 4 B. 0.5 C. 1 D. 0.25 E. 2 Ca(OH)2 which will react completely with 1 mole of nitric acid HNO3. А. 0.5 B. 1 C. 2 D. 0.25 E. 3 3.38. Calculate the number of equivalents of calcium hydroxide Ca(OH)2 which will react completely with 1 mole of orthophosphoric acid H3PO4. А. 0.25 B. 2 C. 3 D. 0.5 E. 1 3.34. Calculate the number of equivalents of aluminium hydroxide Al(OH)3 which will react completely with 1 mole of sulfuric acid H2SO4. А. 0.5 B. 0.25 C. 1 D. 4 E. 2 3.39. Calculate the number of equivalents of aluminium hydroxide Al(OH)3 which will react completely with 1 mole of ortho-phosphoric acid H3PO4. А. 0.25 B. 0.5 C. 2 D. 3 E. 1 3.35. Calculate the number of equivalents of aluminium hydroxide Al(OH)3 which will react completely with 1 mole of nitric acid HNO3. А. 0.25 B. 1 C. 3 D. 2 E. 0.5 3.40. Calculate the number of equivalents of sodium hydroxide NaOH which will react completely with 1 mole of orthophosphoric acid H3PO4. А. 0.25 B. 0.5 C. 1 D. 2 E. 3 3.36. Calculate the number of equivalents of zinc hydroxide Zn(OH)2 which will react completely with 1 mole of nitric acid HNO3. А. 0.25 B. 1 C. 0.5 D. 2 E. 3 3.41. Calculate the number of equivalents of zinc hydroxide Zn(OH)2 which will react 3.37. Calculate the number of equivalents of calcium hydroxide 21 completely with 1 mole of orthophosphoric acid H3PO4. А. 2 B. 1 C. 0.25 D. 0.5 E. 3 B. C. D. E. 1 3 2 5 3.46. For calculation the equivalent mass of sodium oxide Na2O its molar mass should be divided by: А. 2 B. 4 C. 1 D. 3 E. 5 3.42. Calculate the number of equivalents of zinc hydroxide Zn(OH)2 which will react completely with 1 mole of hydrochloric acid HCl. А. 0.5 B. 2 C. 3 D. 1 E. 0.25 3.47. For calculation the equivalent mass of beryllium oxide BeO its molar mass should be divided by: А. 3 B. 5 C. 4 D. 1 E. 2 3.43. Calculate the number of equivalents of aluminium hydroxide Al(OH)3 which will react completely with 1 mole of hydrochloric acid HCl. А. 0.25 B. 2 C. 1 D. 3 E. 0.5 3.48. For calculation the equivalent mass of aluminium oxide Al2O3 its molar mass should be divided by: А. 2 B. 5 C. 6 D. 3 E. 4 3.44. Calculate the number of equivalents of calcium hydroxide Ca(OH)2 which will react completely with 1 mole of hydrochloric acid HCl. А. 3 B. 1 C. 0.25 D. 0.5 E. 2 3.49. For calculation the equivalent mass of iron (III) oxide Fe2O3 its molar mass should be divided by: А. 6 B. 5 C. 4 D. 3 E. 2 3.45. For calculation the equivalent mass of calcium oxide CaO its molar mass should be divided by: А. 4 3.50. For calculation the equivalent mass of chromium (III) oxide Cr2O3 its molar mass should be 22 divided by: А. 6 B. 3 C. 4 D. 2 E. 5 mass of phosphorus (III) oxide P2O3 its molar mass should be divided by: А. 2 B. 5 C. 6 D. 4 E. 3 3.51. For calculation the equivalent mass of sulfur (IV) oxide SO2 its molar mass should be divided by: А. 2 B. 1 C. 4 D. 8 E. 6 3.56. For calculation the equivalent mass of arsenic (V) oxide As2O5 its molar mass should be divided by: А. 5 B. 2 C. 1 D. 3 E. 10 3.52. For calculation the equivalent mass of carbon (IV) oxide CO2 its molar mass should be divided by: А. 4 B. 8 C. 1 D. 6 E. 2 3.57. For calculation the equivalent mass of arsenic (III) oxide As2O3 its molar mass should be divided by: А. 2 B. 5 C. 3 D. 4 E. 6 3.53. For calculation the equivalent mass of sulfur (VI) oxide SO3 its molar mass should be divided by: А. 4 B. 8 C. 6 D. 2 E. 1 3.58. For calculation the equivalent mass of a reactant in the following chemical transformation: HNO3 → NO2, its molar mass should be divided by … А. 1 B. 3 C. 5 D. 4 E. 2 3.54. For calculation the equivalent mass of phosphorus (V) oxide P2O5 its molar mass should be divided by: А. 2 B. 5 C. 10 D. 3 E. 1 3.59. For calculation the equivalent mass of a reactant in the following chemical transformation: H2SO3 → H2SO4, its molar mass should be divided by … 3.55. For calculation the equivalent 23 А. 2 B. 4 C. 5 D. 3 E. 1 B. C. D. E. 3 2 5 4 3.64. For calculation the equivalent mass of a reactant in the following chemical transformation: N2 → NO , its molar mass should be divided by … А. 3 B. 1 C. 5 D. 2 E. 4 3.60. For calculation the equivalent mass of a reactant in the following chemical transformation: S0 → H2S, its molar mass should be divided by … А. 4 B. 3 C. 1 D. 5 E. 2 3.65. For calculation the equivalent mass of a reactant in the following chemical transformation: O2 → CuO , its molar mass should be divided by … А. 4 B. 3 C. 2 D. 1 E. 5 3.61. For calculation the equivalent mass of a reactant in the following chemical transformation: NO → NO2, its molar mass should be divided by … А. 4 B. 3 C. 1 D. 2 E. 5 3.66. For calculation the equivalent mass of sulfuric acid H2SO4 its molar mass should be divided by: А. 4 B. 1 C. 2 D. 3 E. 5 3.62. For calculation the equivalent mass of a reactant in the following chemical transformation: MgO → Mg0 , its molar mass should be divided by … А. 2 B. 5 C. 1 D. 3 E. 4 3.67. For calculation the equivalent mass of hydrochloric acid HCl its molar mass should be divided by: А. 4 B. 2 C. 5 D. 1 E. 3 3.63. For calculation the equivalent mass of a reactant in the following chemical transformation: HNO3 → NO , its molar mass should be divided by … А. 1 24 3.68. For calculation the equivalent mass of phosphoric acid H3PO4 its molar mass should be divided by: А. 4 B. 3 C. 1 D. 5 E. 2 3.73. For calculation the equivalent mass of sodium hydroxide NaOH its molar mass should be divided by: А. 4 B. 5 C. 3 D. 1 E. 2 3.69. For calculation the equivalent mass of acetic acid CH3COOH its molar mass should be divided by: А. 4 B. 2 C. 5 D. 1 E. 3 3.74. For calculation the equivalent mass of potassium hydroxide KOH its molar mass should be divided by: А. 4 B. 2 C. 5 D. 1 E. 3 3.70. For calculation the equivalent mass of nitric acid HNO its molar mass should be divided by: А. 3 B. 2 C. 1 D. 4 E. 5 3.75. For calculation the equivalent mass of calcium hydroxide Ca(OH)2 its molar mass should be divided by: А. 5 B. 4 C. 1 D. 3 E. 2 3.71. For calculation the equivalent mass of perchloric acid HClO4 its molar mass should be divided by: А. 5 B. 2 C. 1 D. 4 E. 3 3.76. For calculation the equivalent mass of barium hydroxide Ba(OH)2 its molar mass should be divided by: А. 2 B. 3 C. 1 D. 5 E. 4 3.72. For calculation the equivalent mass of sulfide acid H2S its molar mass should be divided by: А. 4 B. 5 C. 2 D. 3 E. 1 3.77. For calculation the equivalent mass of magnesium hydroxide Mg(OH)2 its molar mass should be divided by: 25 А. 4 B. 2 C. 5 D. 1 E. 3 mass of calcium carbonate CaCO3 its molar mass should be divided by: А. 4 B. 5 C. 1 D. 3 E. 2 3.78. For calculation the equivalent mass of aluminium hydroxide Al(OH)3 its molar mass should be divided by: А. 4 B. 1 C. 5 D. 2 E. 3 3.83. For calculation the equivalent mass of copper sulfate CuSO4 its molar mass should be divided by: А. 2 B. 5 C. 4 D. 1 E. 3 3.79. For calculation the equivalent mass of sodium chloride NaCl its molar mass should be divided by: А. 1 B. 5 C. 2 D. 4 E. 3 3.84. For calculation the equivalent mass of sodium carbonate Na2CO3 its molar mass should be divided: А. 1 B. 3 C. 2 D. 5 E. 4 3.80. For calculation the equivalent mass of potassium bromide KBr its molar mass should be divided by: А. 2 B. 4 C. 5 D. 1 E. 3 3.85. For calculation the equivalent mass of aluminium chloride AlCl3 its molar mass should be divided by: А. 3 B. 1 C. 5 D. 2 E. 4 3.81. For calculation the equivalent mass of silver nitrate AgNO3 its molar mass should be divided by: А. 1 B. 3 C. 5 D. 4 E. 2 3.86. For calculation the equivalent mass of aluminium bromide AlBr3 its molar mass should be divided by: А. 2 B. 3 C. 5 D. 1 3.82. For calculation the equivalent 26 E. 4 3.89. For calculation the equivalent mass of iron (II) phosphate Fe3(PO4)2 its molar mass should be divided by: А. 5 B. 6 C. 1 D. 2 E. 4 3.87. For calculation the equivalent mass of aluminium nitrate Al(NO3)3 its molar mass should be divided by: А. 2 B. 3 C. 4 D. 1 E. 5 3.90. For calculation the equivalent mass of iron (III) sulfate Fe2(SO4)3 its molar mass should be divided by: А. 3 B. 6 C. 2 D. 4 E. 5 3.88. For calculation the equivalent mass of calcium phosphate Ca3(PO4)2 its molar mass should be divided by: А. 3 B. 1 C. 5 D. 2 E. 6 Chapter 4. Solutions. Ways of expressing concentration of solutions 4.1. What shows a curve of solubility of a gas? А. dependence solubility of a gas with pressure B. dependence solubility of a gas with temperature C. dependence solubility of a gas with presence of another substances D. dependence solubility of a gas with nature of compound E. dependence solubility of a gas with nature of solvent 4.2. How changes the solubility of a gas with increasing a pressure? А. decrease B. increase proportionally C. decrease proportionally D. does not change E. спадає 4.3. How changes the solubility of a gas with decreasing a pressure? А. decrease B. decrease proportionally C. increase proportionally D. solubility doesn’t depend on 27 pressure E. does not change B. number of moles of solute that are in 1kg of solution C. number of moles of solute that are in 100сm3 of solution D. number of moles of solute that are in 1сm3 of solution E. number of moles of solute that are in 1L of solution 4.4. How changes the solubility of a gas with increasing a temperature? А. does not change B. increase proportionally C. decrease D. E. increase 4.9. Molality – that is: А. number of moles of solute that are in 1сm3 of solution B. number of moles of solute that are in 100сm3 of solution C. number of moles of solute that are in 1L of solution D. number of moles of solute that are in 1kg of solvent E. number of moles of solute that are in 1kg of solution 4.5. How changes the solubility of a gas with decreasing a temperature? А. does not change B. increase proportionally C. increase D. decrease E. 4.6. How changes the solubility of a oxygen in a blood with increasing a pressure? А. increase B. decrease proportionally C. decrease D. E. does not change 4.10. Normality (molar concentration of equivalent) – that is: А. number of mol-equivalen of solute that are in 1L of solution B. number of mol-equivalen of solute that are in 100 сm3of solution C. number of mol-equivalen of solute that are in 1kg of solution D. number of mol-equivalen of solute that are in 1 cm3of solution E. number of mol-equivalen of solute that are in 1kg of solvent 4.7. Mass percentage of solute – that is: А. relation between number of solute and mass of solvent B. relation between mass of solute and volume of solution C. number of moles of solute that are in 100g of solution D. gram of solute in 1000g of solution E. the percentage by mass of solute contained in a solution 4.11. Titr – that is: А. number of gram of solute in 1 L of solution B. number of gram of solute in 1 сm3 of solvent C. number of gram of solute in 1 4.8. Molarity – that is: А. number of moles of solute that are in 1kg of solvent 28 cm3 of solution D. number of gram of solute in 1 g of solvent E. number of gram of solute in 1 kg of solution E. 15 4.16. How many grams of NaCl are required to prepare 50 g of 10% hypertonic solution? А. 50 g B. 5 g C. 10 g D. 0,5 g E. 1 g 4.12. Mole fraction of a solute – that is: А. moles of solute divided by the moles of solvent B. relation between number of particles in a system solute and mass C. moles of component substance divided by the total moles of solution D. moles of solvent divided by the total moles of solution E. moles of solvent divided by the moles of solute 4.17. How many grams of glucose are required to prepare 200 g of 4% hypotonic solution? А. 20 g B. 8 g C. 200 g D. 2 g E. 80 g 4.13. How many grams of AgNO3 are required to prepare 10 g of 2% solution? А. 10 B. 20 C. 2 D. 0,2 E. 0,1 4.18. How many grams of glucose are required to prepare 200 g of 5% isotonic solution? А. 5 g B. 100 g C. 20 g D. 50 g E. 10 g 4.14. How many grams of NaCl are required to prepare 200 g of 0,9 % solution? А. 1,8 B. 0,9 C. 18 D. 0,18 E. 9 4.19. What is the mass percentage (%) of C12H22O11 in a solution that contains 30.0 g of C12H22O11 in 570 g of water? А. 10 B. 20 C. 5 D. 15 E. 30 4.15. How many grams of glucose are required to prepare 500 g of 5% solution? А. 25 B. 5 C. 50 D. 100 4.20. What is the mass percentage (%) of glucose in a solution that contains 20 g of glucose in 180 g of water? А. 10 B. 15 29 C. 5 D. 30 E. 20 B. C. D. E. 1,83 1,67 1,25 1,00 4.21. What is the mass percentage (%) of glicerine in a solution that contains 40.0 g of glicerine in 360 g of water? А. 40 B. 10 C. 15 D. 30 E. 20 4.26. What is the molarity of a solution containing 5,6 g of KOH in 400 ml of solution? А. 1,00 B. 0,75 C. 0,10 D. 0,25 E. 0,50 4.22. What is the molarity of a solution containing 4.0 g of NaOH in 500 ml of solution? А. 0,15 B. 0,2 C. 0,1 D. 0,3 E. 0,25 4.27. What is the molarity of a solution containing 56 g of KOH in 500 ml of solution? А. 2,0 B. 0,5 C. 2,5 D. 1,0 E. 1,5 4.23. What is the molarity of a solution containing 40.0 g of NaOH in 800 ml of solution? А. 2,50 B. 1,25 C. 1,00 D. 1,50 E. 0,50 4.28. What are the mole fraction of a solution containing 2 mole of NaOH and 18 mole Н2О? А. 2,0 B. 0,1 C. 0,01 D. 0,02 E. 0,2 4.24. What is the molarity of a solution containing 9.8 g of H2SO4 in 200 ml of solution? А. 0,50 B. 0,75 C. 1,00 D. 0,25 E. 0,10 4.29. What are the mole fraction of a solution containing 2 mole of glucose and 18 mole Н2О? А. 1,0 B. 0,1 C. 2,0 D. 0,2 E. 0,01 4.25. What is the molarity of a solution containing 98 g of H2SO4 in 600 ml of solution? А. 1,50 4.30. What are the mole fraction of a solution containing 4 mole of glucose and 36 mole Н2О? А. 0,1 30 B. C. D. E. C12H22O11 and 45 mole Н2О? А. 0,5 B. 0,05 C. 0,1 D. 5 E. 0,01 2,0 1,0 0,4 4 4.31. What are the mole fraction of a solution containing 5 mole of Chapter 5. Colligative properties of solutions 5.1. Colligative properties depend on the: А. nature of solvent B. number of solute and solvent molecules (or ions) C. percent of ionization D. nature of solute E. temperature 5.4. Osmotic concentration (osmolarity)– that is: А. total concentration of solute and solvent molecules in a solution B. mass percentage of electrolytes C. molar concentration of albumen molecules D. concentration of electrolytes E. concentration of albumen molecules 5.2. Colligative properties are: А. osmosis B. diffusion C. osmosis and diffusion D. solubility E. – 5.5. Solutions, that have the same osmotic pressure are: А. hypotonic B. isotonic C. hypertonic D. concentrated E. saturated 5.3. Oncotic preassure – is: А. total osmotic preassure of biological liquids B. C. osmotic preassure that is caused by electrolytes in biological liquids D. osmotic preassure that is caused by albumen molecules, dissolved in a biological liquids E. osmotic preassure that is caused by iones in biological liquids 5.6. Value of an oncotic pressure (кPa) are: А. 3.5·102 – 3.9·103 B. 0.35 – 0.39 C. 3.5·102 – 3.9·102 D. 3.5 – 3.9·102 E. 3.5 – 3.9 5.7. Value of an osmotic pressure 31 (кPa) of a blood: А. 7.4·103 – 7,8·102 B. 7.4·102 – 7,8·102 C. 7,4 – 7,8 D. 7.4·10 – 7,8·102 E. 74 – 78 D. 0,9% NaCl and 0,5% glucose E. 0,9% NaCl and 5%-glucose 5.13. What happen with red blood cells, when they are placed in a 10% NaCl solution? А. B. crenation C. hemolysis D. does not change E. hemolysis and crenation 5.8. What happen with red blood cells, when they are placed in a hypertonic solution? А. diffusion B. hemolysis and crenation C. D. hemolysis E. crenation 5.14. What happen with red blood cells, when they are placed in a 1% glucose solution? А. does not change B. C. hemolysis and crenation D. crenation E. hemolysis 5.9. What happen with red blood cells, when they are placed in a hypotonic solution? А. diffusion B. hemolysis C. hemolysis and crenation D. osmosis E. crenation 5.15. What happen with red blood cells, when they are placed in a 5% glucose solution? А. diffusion B. hemolysis and crenation C. does not change D. crenation E. hemolysis 5.10. How to calculate osmotic pressure of electrolytes solution? А. π = iCNRT B. π = νRT C. π = iCmRT D. π = CMRT E. π = iCMRT 5.16. Freezing point depression constant depend on the: А. concentration of solution B. nature of solvent C. nature of solute D. temperature E. presence of catalyst 5.11. How to calculate osmotic pressure of non-electrolytes solution? А. π = CMRT B. π = iCMRT C. π = CNRT D. π = iCNRT E. π = (m/M)RT 5.17. What is the freezing point of solution (0С) containing 1 mol of maltose and 2000 g of water? А. –0.93 B. –1.86 C. +1.86 D. –3.72 5.12. Isotonic solutions are: А. 5% NaCl and 0,9% glucose B. 9% NaCl and 5% glucose C. 9% NaCl and 0,5% glucose 32 E. +3.72 C. 100.52 D. 100.00 E. 101.,86 5.18. Boiling point elevation constant depend on the: А. presence of catalyst B. nature of solvent C. nature of solute D. temperature E. concentration of solution 5.20. What is the boiling point of solution (0С) containing 1 mol of urea and 500 g of water? А. 101.04 B. 100.00 C. 101.00 D. 100.52 E. 101.86 5.19. What is the boiling point of solution (0С) containing 2 mol of glucose and 2000 g of water? А. 101. 04 B. 101.00 Chapter 6. The basic terms of chemical thermodynamics. Thermochemistry. The direction of chemical processes passage B. T=const, U=const C. P=const, U=const D. V=const, Q=const E. P=const, T=const 6.1. The mass of the substance (m) and its volume (V) are: А. intensive properties B. state functions of the system C. thermodynamic potentials D. thermodynamic properties E. extensive properties 6.4. Thermochemistry is the study of: А. transformations between different kinds of matter B. different kinds of energy changing associated with physical and chemical processes C. transformations of internal energy into work under chemical reactions D. different kinds of energy changing associated with chemical reactions E. transformations of heat into internal energy under chemical 6.2. Temperature (Т) and pressure (Р) are: А. kinetic properties B. extensive properties C. thermodynamic potentials D. intensive properties E. state functions of the system 6.3. The isothermal – isochoric thermodynamic process is carried out under: А. T=const, V=const 33 reactions according to the way of their interaction with the surroundings: А. homogeneous and heterogeneous B. isolated, closed, open C. physical and chemical D. one-, two- and threecomponents E. equilibrium and nonequilibrium 6.5. The isothermal – isobaric thermodynamic process is carried out under: А. T=const, U=const B. P=const, U=const C. T=const, V=const D. T=const, P=const E. V=const, P=const 6.6. A system which can exchange energy only with the surroundings is called: А. isolated B. equilibrium C. open D. closed E. thermodynamic 6.10. Chemical thermodynamics is based on: А. the main laws of physics and chemistry B. two main laws of thermodynamics C. three main laws of thermodynamics D. different physical laws and equations E. one main laws of thermodynamics 6.7. What is the kind of a system when metal plate of zinc is dipped into the test-tube with the sulfuric acid H2SO4 solution? А. physical, heterogeneous, closed B. chemical, heterogeneous, open C. chemical, heterogeneous, isolated D. chemical, homogeneous, isolated E. chemical, homogeneous, open 6.11. State functions are the parameters of systems which depend on: А. the initial and final states of the system B. the ability of a system to come back to its initial state C. the path by which the process occurs D. the equilibrium constant value E. the exchange by mass and energy with the surroundings 6.8. What is the kind of a system when calcium carbonate CaCO3 is heated in a test-tube? А. heterogeneous, two-phases B. heterogeneous, equilibrium C. heterogeneous, three-phases D. heterogeneous, nonequilibrium E. homogeneous, one-phase 6.12. Heat and work depends on: А. the initial and final states of the system B. the exchange by mass and energy with the surroundings C. the ability of a system to come back to its initial state D. the equilibrium constant value E. the path by which the process 6.9. Thermodynamic systems may be divided into following types 34 occurs А. 1, 2, 3 B. 1, 3 C. 3 D. 2 E. 1 6.13. Does the ∆Н°298 value for a chemical reaction depends on the presence of a catalyst in a system? А. depends of nature of a catalyst B. doesn’t depend C. depends in homogeneous systems D. depends E. depends in heterogeneous systems 6.18. Which reactions through the listed ones are endothermic? 1. N2 + O2 = 2NO 2. H2 + Cl2 = 2HCl 3. H2 + S = H2S А. 1, 2, 3 B. 1 C. 2, 3 D. 3 E. 2 6.14. The main law of thermochemistry is: А. Hess’ law B. Nernst’ law C. Henry’s law D. Avogadro’ law E. law of mass action 6.19. Point out the correct consequence of the thermal stability increasing for hydrogen halogenides. The standard enthalpies of formation values for hydrogen halogenides (in kJ/mol) are: ∆H°(HBr) = – 36,3; ∆H°(HI) = 26,6; ∆H°(HF) = −270,7; ∆H°(HCl) = −92,3. А. HF < HBr < HCl < HI B. HI > HBr > HCl > HF C. HI < HBr < HCl < HF D. HF < HCl < HBr < HI E. HBr < HCl < HF < HI 6.15. The standard enthalpy of formation for which of the following substances is equal to zero? А. O3 B. О2 C. СО2 D. Н2О E. С6Н12О6 6.20. Which compound through the listed ones can be more easily decomposed under heating? А. N2O3(g) B. all compounds are readily decompose identically C. N2О(g) D. NO(g) E. NO2(g) 6.16. The standard enthalpy of formation for which of the following substances is equal to zero? А. H2O(g) B. HF(g) C. HF(liquid) D. N2(solid) E. N2 (g) 6.21. What will be the standard enthalpy change for the reaction: С(graphite) + 2 Н2(g) = СН4(g)? А. it will increase at 75 kJ B. it will decrease at 51 kJ 6.17. Which reactions through the listed ones are exothermic? 1. N2 + O2 = 2NO 2. H2 + Cl2 = 2HCl 3. H2 + I2 = 2HI 35 C. it will decrease at 175 kJ D. it will increase at 186 kJ E. it will decrease at 75 kJ the internal energy of the system: Q= ∆U? А. for equilibrium processes B. for isochoric processes C. for isobaric processes D. for adiabatic processes E. for isothermal processes 6.22. The mathematical equation for the first law of thermodynamics is: А. Q = ∆U + W B. ∆U = Q – W C. Q = ∆U – W D. Q = – ∆Н E. ∆U = Q + W 6.27. For what kind of thermodynamic processes the heat effect of the reaction equals to the change of the enthalpy: Q = ∆H? А. for isobaric processes B. for equilibrium processes C. for isochoric processes D. for adiabatic processes E. for isothermal processes 6.23. For which of the given substances the standard enthalpy of formation doesn’t equal to zero? А. С(diamond) B. I2(solid) C. Р(white) D. N2(g) E. С(graphite) 6.28. The heat effect for the process of water transformation from liquid to steam equals to: А. - 44 kJ/mol B. 4.4 kJ/mol C. 40 kJ/mol D. 44 kJ/mol E. -241.8 kJ/mol 6.24. 10.5 kJ of heat released when 11.2 L of H2S was formed. What is the standard enthalpy of formation for H2S? А. 10.5 kJ/mol B. –21.0 kJ/mol C. 21.0 kJ/mol D. 5.25 kJ/mol E. –5.25 kJ/mol 6.29. For which of the listed compounds the standard enthalpies of combustion (∆Н°comb.) are equal to zero? А. СО, NН3 B. NO, NH3 C. О2, Н2 D. СО2, Н2О E. СО, Н2 6.25. Which reactions through the listed ones are exothermic: 1. N2O4 = 2NO2 2. 1/2 N2 + 1/2 O2 = NO 3. H2 + 1/2 O2 =H2O А. 2 B. no one through the listed reactions C. 1 D. 1, 2, 3 E. 3 6.30. In which of the given cases a reaction is possible at any temperatures? А. ∆H0 > 0 і ∆S0 < 0 B. |∆H0| = |T∆S0| C. ∆H0 < 0 і ∆S0 > 0 D. no one 6.26. For what kind of thermodynamic processes the heat effect of the reaction equals to the change of 36 E. ∆H0 > 0, T=0 FeCl3(solid) B. CO(g) + Cl2(g) = COCl2(g) C. 4 Al(solid) + 3 C(solid) = Al4C3(solid) D. CH4(g) + Cl2(g) = CH3Cl(liquid) + HCl(g) E. 2 NH3(g) = N2(g) + 3 H2(g) 6.31. Point out for which of the given processes ∆S0 > 0. А. 2H2S(g) + 3O2(g) → 2H2O(g) + 2SO2(g) B. for none of the given reactions C. H2(g) + F2(g) → 2HF(g) D. NH4NO2(solid) → 2H2O(g) + N2(g) E. 2CO(g) + O2(g) → 2CO2(g) 6.36. The change of free Gibbs’ energy may be calculated as: А. F = U – TS B. ∆G = T∆S – ∆U C. ∆G = ∆H – T∆S D. ∆G = T∆S – ∆H E. G = H + T∆S 6.32. Point out for which of the given processes the entropy increases? А. water freezing B. ethylene polymerization C. association of ions D. evaporation of alcohol E. transformation of graphite into diamond 6.37. Free Gibbs’ energy is the measure of: А. internal energy B. dispersal energy C. non-reversible process D. the energy which can be used for fulfilling a work E. thermodynamic stability of the system 6.33. What is the standard entropy change for the process of graphite transformation into diamond? SС(diamond)= 2,4 J/(mol•К)? А. - 8.1 J/(mol•К) B. 5.7 J/(mol•К) C. 8.1 J/(mol•К) D. -3.3 J/(mol•К) E. 3.3 J/(mol•К) 6.38. Point out without any calculations for which of the given processes the entropy increases? А. 4 HСl(g) + О2(g) = 2Cl2(g) + 2 H2O(g) B. 2СО(g) + О2(g) = 2СО(g) C. С(graphite) + СО2(g) = 2СО(g) D. 2SO2(g) + 2NO2(g) = 2SO2(g) + N2(g) E. H2(g) + Сl2(g) = 2HCl(g) 6.34. The entropy of elements may be equal to zero only under the conditions of: А. normal conditions B. the temperature of absolute zero (T=0 °K) C. STP D. P = const E. T = const 6.39. A chemical reaction is impossible at any temperature in the case of: А. ∆H < 0, ∆S < 0, ∆G < 0 B. ∆H < 0, ∆S < 0, ∆G > 0 C. ∆H > 0, ∆S < 0, ∆G > 0 D. ∆H < 0, ∆S > 0, ∆G > 0 6.35. Point out without any calculations for which of the given processes the entropy increases? А. 2 Fe(solid) + 3 Cl2(g) = 2 37 E. ∆H > 0, ∆S > 0, ∆G > 0 compounds in the organism? А. CO2, N2, H2O B. CO, H2O, SO3 C. CO, NO, H2O D. CO2, NO2, SO3 E. CO2, NO, SO2 6.40. Is it possible to determine the absolute value of the enthalpy and free Gibbs’energy? А. possibly to determine at STP B. possibly to determine at normal conditions C. it is possibly to determine experimentally D. impossible E. possible 6.45. What kind of system are biological systems? А. homogeneous closed B. heterogeneous nonequilibrium C. homogeneous open D. heterogeneous isolated E. homogeneous equilibrium 6.41. Entropy is the measure of: А. internal energy B. heat capacity of the system C. the energy which can be used for fulfilling a work D. dispersal energy E. reservation of energy of a system 6.46. What kind of system are the living organism cells? А. open B. non-equilibrium C. equilibrium D. closed E. isolated 6.42. Is the forward reaction C(graphite) + CO2(g) = 2 CO(g) possible? At what conditions? А. possible at steady pressure B. possible at high temperature C. possible at normal conditions D. impossible even at high temperature E. possible at STP 6.47. The main source of energy for the human’s organism is: А. ATP B. carbohydrates C. proteins D. vitamins and minerals E. fats 6.43. The decreasing of Gibbs’ free energy shows that the forward reaction is spontaneous at the conditions of: А. m = constant, heat capacity is const B. P = const C. P = const, V = const D. T = const, P = const E. V = const, T = const 6.48. The daily ration of proteins for an adult is: А. 50–80 g B. 150-200 g C. 60–70 g D. 75–85 g E. 30–50 g 6.49. The daily ration of fats for an adult is: А. 70–80 g B. 60–70 g C. 110–120 g 6.44. What substances are the final products of metabolism of organic 38 D. 30–50 g E. 90-110 g 6.53. What kind of thermodynamic system is the living organism? А. opened system in the state of thermodynamic equilibrium B. closed system in stationary state C. closed system in the equilibrium state D. opened system in stationary state E. isolated system in the equilibrium state 6.50. The daily ration of carbohydrates for an adult is: А. 60–70 g B. 90–100 g C. 250–280 g D. 300–370 g E. 380–390 g 6.51. Energy which is accumulated in the organism may be released under the process of: А. photosynthesis B. bio-polymers synthesis C. foods metabolism D. enzyme catalysis E. ATP hydrolysis 6.54. Which one of the given equations is the expression of the second law of thermodynamics for biological systems? А. S = Smax B. ∆Q = T ⋅ ∆S C. ∆S > 0 6.52. The molecules of what compounds can act as energy storage in bio-systems? А. DNA B. RNA C. АDP D. ATP E. glucose ∆τ D. ∆S = ∆Q T E. ∆S = 0 Chapter 7. The rate and mechanism of chemical reactions 7.2. Сhose the reaction from the given ones that has the highest rate: t → А. MgCO3 MgO + CO2 + B. H2O + H = H3O+ C. 2SO2 + O2 = 2SO3 D. H2 + I2 = 2HI E. 3H2 + N2 = 2NH3 7.1. The units of the rate of a chemical reaction in homogeneous systems? А. mol⋅m2⋅min–1 B. mol⋅L–1⋅min. C. mol⋅м3⋅s–1 D. mol⋅L–1⋅s–1 E. mol⋅mL–1⋅s–1 7.3. Сhose the reaction from the given 39 ones that has the highest rate: Kt А. C2H12O6 → 2C2H5OH + 2CO2 Kt B. CO + 2H → CH3OH 2 t C. → 2NH3 N2 +3H2 D. NaOH + HCl = NaCl + H2O E. 2NO + Cl2 = 2NOCl is: А. B. C. D. E. 7.8. Chose the reaction that take place with the highest rate, if for the same interval of time 3,0 grams of products were forms? 7.4. The expression of the Rate Law for reaction 2NO (g)+O2(g) = 2NO2(g) is: А. v = [2NO] 2 ·[O2] B. v =k[NO]2·[O2] C. v =k[NO] + [O2] D. v =k[NO2]2·[O2] E. v = [NO]2 + [O2] I. 1 2 1 2 Cl2(g) = HCl(g) O2(g) = H2O(g) 1 7.9. Сhose the reaction from the given ones that has the highest rate: А. 2SO2+ O2 = 2SO3 B. Ва(OH)2 + 2HCl = ВаCl2 + 2H2O C. (C6H10O5)n + nH2O + ,t H → nC6H12O6 ∆τ ∆c υ =− ∆τ C. c −c υ= 2 1 τ 2 − τ1 D. B. υ =± H2(g) + 1 υ= E. 2 III. 2 H2(g) + 2 F2(g) = HF(g) А. B. rate is the same C. II D. I E. III 7.6. The instantaneous rate may be calculated according to equation: А. ∆c υ = kc 2 1 II. H2(g) + 7.5. The units of the rate of a chemical reaction in heterogeneous systems: А. molּmLּs-1 B. molּLּs-1 C. kmolּм3ּmin-1 D. mol/(cm3ּs) E. molּL/min D. v = k[SO2] + [O2] v = k[SO2]2 [O2] v = [SО2] [O2] v = [SO2] [O2]2 v = k[SO3] 2NH3 t → N2 + 3H2 Kt E. 2C H OH → (C2H5)2O + 2 5 H2O 7.10. The expression of the Rate Law for reaction 2CO(g) + O2(g) = 2CO2(g) is: А. v = k[CO]2⋅[O2] B. v = k[CO2]2 C. v = [CО]⋅ [O2] D. v = [CO]2⋅[O2] E. v = k[2CO] ⋅[O2] dc dτ 7.7. The expression of the Rate law for reaction 2SO2(g) + O2(g) = 2SO3(g) 40 СаСО3(s) → СаО(s) + СО2(g) correct expression of the rate law is: А. v = k[СаО] + [СO2] B. v = k[СаО ][СO2] C. v = k[СаСО3 ] D. v = k E. v = [СаСО3 ] 7.11. The rate increases in a heterogeneous systems with: А. increasing surface area B. decreasing of concentration of reactant C. irradiation D. all methods E. cooling 7.17. Decomposition of HI (hydrogen iodide) on the surface of gold is the reaction of: А. first order B. higher order C. zero order D. E. second order 7.12. The expression of the Rate Law for reaction С(gr) + СО2(g) = 2СО(g) is: А. v = k[С] [СO] B. v = k [С] [СO2] C. v = k[С] D. v = k [СO2] E. v = k[СО2] 7.18. Decomposition of NH3 on the surface of tungsten (W) is the 7.13. The expression of the Rate Law for reaction Н2О(g) + СО(g) = СО2(g) +Н2(g) is: А. v = k[Н2О ] [СO] B. v = k ([Н2О ] + [СO]) C. v = k [Н2 ] [СO2] D. v = k [СO] E. v = [Н2О ] [СO] reaction of: 2NH3 +3H2 А. higher order B. second order C. first order D. zero order E. - 7.14. The expression of the Rate Law for reaction N2(g) + 3Н2(g) = 2NH3(g) is: А. v = k[N2 ] [H2]3 B. v = k[N2 ]+ [H2]3 C. v = k[NH3 ]2 D. v = 2[N2 ] 3[H2] E. v = [N2 ] [H2]3 t →N 2 7.19. The given reaction of hydrolysis С12Н22О11 + Н2О = С6Н12О6 (gl) + С6Н12О6(fr) is: А. bimolecular reaction of the zero order B. monomolecular reaction of the second order C. bimolecular reaction of the first order D. bimolecular reaction of the second order E. monomolecular reaction of the first order 7.15. How to name the main law of the chemical kinetics? А. Rate law B. Ostwald’s law C. D. The law of equivalents E. Henry’s law 7.20. The reaction CO + Cl2 = COCl2 is a reaction: 7.16. According to the reaction 41 А. bimolecular reaction of the second order B. monomolecular reaction of the first order C. bimolecular reaction of the first order D. monomolecular reaction of the second order E. monomolecular reaction of the zero order Ea in the most of chemical reactions: А. 100-300 kJ/mol B. 150-300 kJ/mol C. 50-150 kJ/mol D. 100-200 kJ/mol E. 40-200 kJ/mol 7.25. Which of the given reactions take place by the molecular mechanisms А. 2NO + O2 = 2NO2 B. 2H2 + O2 = 2H2O C. H3O+ + OH– = 2H2O D. H2 + Cl2 = 2HCl E. Ba2+ + SO42– = BaSO4 7.21. The reaction CuO(s) + H2(g) = Cu(s) + H2O(l) is a reaction: А. bimolecular reaction of the first order реакція B. bimolecular reaction of the second order C. heterogeneous, second order D. monomolecular reaction of the first order E. homogeneous, second order 7.26. Which of the given reactions take place by the radical mechanisms? А. CH4 + Cl2 = CH3Cl + HCl B. HNO3 + KOH = KNO3 + H2O C. 2NO + O2 = 2NO2 D. AgNO3 + HCl = AgCl↓ + HNO3 E. H2 + I2 = 2HI 7.22. When the value of rate constant and rate of reaction are equal? А. reaction take place in homogeneous system B. reaction take place in homogeneous system C. concentrations of reactants are equal 1 mol/L D. concentrations of reactants are the same, but not equal 1 mol/L E. in all cases 7.27. If the activation energy of the chemical reaction is Еа > 120 kJ/mol, its rate is: А. high B. very high C. average D. low E. very low 7.23. When the order and the molecularity are congruent? А. for photochemical reaction B. for complicated reaction C. always are congruent D. E. only for simple, one step reaction 7.28. If the activation energy of the chemical reaction is Еа ≈ 40 kJ/mol, its rate is: А. very low B. low C. high D. very high E. average 7.24. The range of the activation energy 7.29. The solubility of a slightly 42 dissolved salt AmBn, which dissociate according to the scheme → AmBn ← mAn+ + nBm– may be calculated as: А. Ksp S= form?(IP – ion product) А. ІP = Ksp B. ІP> Ksp C. ІP ≈ Ksp D. E. ІP< Ksp m+n 7.34. Solubility product constant Ksp depends on: А. B. presence of catalyst C. molar concentration of ions D. concentration of salt E. temperature B. S = K sp C. S= (m⋅n) D. S= (m+n) E. S= (m+n) Ksp m⋅n Kp m+n Ksp 7.35. The solubility product constant Ksp of a slightly dissolved salt AmBn, which dissociate according → to the scheme AmBn ← mAn+ + nBm– may be calculated as: А. Ksp = [A]m⋅[В]n B. Ksp = [An+]m⋅[Bm–]n C. a n+ ⋅ aBmKsp = A D. a n + + a B mKsp = A E. Ksp = [An+]⋅[Bm–] mm ⋅ nn 7.30. What is the relations between Gibbs energy and the solubility product constant Ksp? А. log Ksp =∆G/(2,303RT) B. ∆G = - RTln Ksp C. ∆G = RTlog Ksp D. ∆G = RTln Ksp E. log Ksp =–∆G/(2,303RT) ( 7.31. In which case the solution of BaSO4 is over-saturated? А. [Ba2+]⋅[SO42–] = Ksp B. [Ba] ⋅[SO4]= Ksp C. [Ba2+]⋅[SO42–] > Ksp D. [Ba2+]⋅[SO42–] ≈ Ksp E. [Ba2+]⋅[SO42–] < Ksp ) ( ) 7.36. The solubility of a slightly dissolved salt is expressed by the: А. dissociation constant B. coefficient of absorption C. solubility product constant D. percent of solubility E. coefficient of solubility 7.32. In which case the solution of AgI is saturated? А. [Ag+]⋅[I–] < Ksp B. [Ag+]⋅[I–]≈ Ksp C. [Ag+]⋅[I–] > Ksp D. [Ag+]⋅[I–] = Ksp E. [Ag+]+[I–] < Ksp 7.37. The expression of the solubility product constant Ksp for Ca3 (PO4)2 is: А. Ksp = 3[Ca2+]3· 2[PO43–]2 B. Ksp = [Ca2+]3·[PO43–]2 C. Ksp = 3[Ca2+]3+2[PO43–]2 D. Ksp = ([Ca2+]3·[PO43–]2)/ [Ca3(PO4)2] 7.33. In which case the precipitate of slightly dissolved salt will 43 E. Ksp = [Ca2+]3 + [PO43–]2 D. Ksp = a(Ca2+) a(F–) E. Ksp = [Ca2+][2F–] 7.38. Compare the values for Ksp next salts HgS, PbS, CdS, SnS, TlS2 and choose one, which is the least soluble in a water. А. Ksp (HgS) = 4,0·10–53 B. Ksp (TlS2) = 5·10–21 C. Ksp (PbS) = 2,5·10–27 D. Ksp (CdS) = 1,2·10–28 E. Ksp (SnS) = 1,0·10–27 7.43. The expression of the solubility product constant Ksp for Ag2CrO4: А. Ksp = [Ag+]+[Cr2O42–] B. Ksp = 2[Ag+]•[CO42–] C. Ksp = [Ag+]2⋅[CrO42–] D. Ksp = [Ag+]⋅[CrO42–] 2 E. (a ) ⋅ aCrO Ksp = Аg + 2- 4 7.44. The solubility of Ag2S (in mol/L) may be calculated according to the equation: А. S= K sp 7.39. Which ions can not be present simultaneous (одночасно) in a solution? А. Na+, SO42– B. Cu2+, SO42– C. Fe2+, OH– D. NH4+, Cl– E. K+, NO3– B. S= 3 Ksp 4 C. S= 3 4K sp 7.40. Choose the pair of compound, which ions can not be present simultaneous in a solution: А. Ba(OH)2 and CO2 B. NaOH and P2O5 C. AgNO3 and HCl D. Al(NO3)3 and HCl E. CuSO4 and BaCl2 D. Ksp S= 2 E. S= 3 Ksp 27 7.45. The solubility of Fe(OH)3 (in mol/L) may be calculated according to the equation: А. Ksp S= 3 4 B. S= 3 4K 7.41. The expression of the solubility product constant Ksp for Mg(OH)2: А. = aMg 2+ ⋅ a _ OH Ksp 2+ B. Ksp = [Mg ]⋅[OH–] C. Ksp = [Mg2+]⋅[OH–]2 D. Ksp = [Mg2+]•2[OH–] E. Ksp = 2[Mg2+]•2[OH–] sp C. S= 3 Ksp 27 D. S= K sp E. 7.42. The expression of the solubility product constant Ksp for CaF2: А. Ksp = [Ca2+] + [F–] B. Ksp = [Ca2+][F–]2 C. Ksp = [Ca2+] / [F–]2 S= 4 K sp 27 7.46. Expression of the equilibrium constant for the reaction 2SO2 + O2 ⇄ 2SO3 is: 44 [SO 2 ]2 ⋅ [O 2 ] [SO 3 ]2 [SO3 ] B. Kp = [SO 2 ] ⋅ [O 2 ] А. Kp = [SO3 ]2 ⋅ [O 2 ] [SO 2 ]2 C. Kp = [SO 2 ]2 [SO3 ]2 ⋅ [O 2 ] D. А. C. D. E. Kp = Kp = B. Kp Kp Kp 2 [SO3 ] [SO 2 ]2 ⋅ [O 2 ] E. [H 2 ] [H 2 O] [FeO] ⋅ [H 2 ] = [Fe] ⋅ [H 2 O] [H О] = 2 [H 2 ] [H 2 O] ⋅ [Fe] = [H 2 ] ⋅ [FeO] [H 2 O] = [H 2 ] ⋅ [FeO] Kp = Kp 7.47. Expression of the equilibrium constant for the reaction FeO(s) + H2(g) ⇄ Fe(s) + H2O(g) ? Chapter 8. The equilibrium in feebly soluble electrolytes solutions product constant Ksp? А. log Ksp =–∆G/(2,303RT) B. ∆G = - RTln Ksp C. ∆G = RTln Ksp D. ∆G = RTlog Ksp E. log Ksp =∆G/(2,303RT) 8.1. The solubility of a slightly dissolved salt AmBn, which dissociate according to the scheme AmBn ⇄ mAn+ + nBm– may be calculated as: А. (m+n) Ksp S= 8.3. In which case the solution of BaSO4 is over-saturated? А. [Ba2+]⋅[SO42–] < Ksp B. [Ba2+]⋅[SO42–] ≈ Ksp C. [Ba2+]⋅[SO42–] > Ksp D. [Ba] ⋅[SO4]= Ksp E. [Ba2+]⋅[SO42–] = Ksp mm ⋅ nn B. S = K sp C. S= Ksp m+n D. S= (m+n) E. S= (m⋅n) Kp m+n 8.4. In which case the solution of AgI is saturated? А. [Ag+]⋅[I–] > Ksp B. [Ag+]⋅[I–] < Ksp C. [Ag+]+[I–] < Ksp Ksp m⋅n 8.2. What is the relations between Gibbs energy and the solubility 45 А. Ksp = 3[Ca2+]3· 2[PO43–]2 B. Ksp = [Ca2+]3 + [PO43–]2 C. Ksp = 3[Ca2+]3+2[PO43–]2 D. Ksp = ([Ca2+]3·[PO43–]2)/ [Ca3(PO4)2] E. Ksp = [Ca2+]3·[PO43–]2 D. [Ag+]⋅[I–] = Ksp E. [Ag+]⋅[I–]≈ Ksp 8.5. In which case the precipitate of slightly dissolved salt will form?(IP – ion product) А. ІP ≈ Ksp B. ІP< Ksp C. D. ІP = Ksp E. ІP> Ksp 8.10. Compare the values for Ksp next salts HgS, PbS, CdS, SnS, TlS2 and choose one, which is the least soluble in a water. А. Ksp (TlS2) = 5·10–21 B. Ksp (PbS) = 2,5·10–27 C. Ksp (CdS) = 1,2·10–28 D. Ksp (HgS) = 4,0·10–53 E. Ksp (SnS) = 1,0·10–27 8.6. Solubility product constant Ksp depends on: А. B. molar concentration of ions C. temperature D. concentration of salt E. presence of catalyst 8.11. Which ions can not be present simultaneous (одночасно) in a solution? А. K+, NO3– B. Cu2+, SO42– C. NH4+, Cl– D. Na+, SO42– E. Fe2+, OH– 8.7. The solubility product constant Ksp of a slightly dissolved salt AmBn, which dissociate according → to the scheme AmBn ← mAn+ + nBm– may be calculated as: А. a n + + a B mKsp = A B. Ksp = [An+]m⋅[Bm–]n C. Ksp = [An+]⋅[Bm–] D. Ksp = [A]m⋅[В]n E. a n+ ⋅ aBmKsp = A ( ) ( 8.12. Choose the pair of compound, which ions can not be present simultaneous in a solution: А. AgNO3 and HCl B. CuSO4 and BaCl2 C. NaOH and P2O5 D. Al(NO3)3 and HCl E. Ba(OH)2 and CO2 ) 8.8. The solubility of a slightly dissolved salt is expressed by the: А. percent of solubility B. solubility product constant C. coefficient of absorption D. coefficient of solubility E. dissociation constant 8.13. The expression of the solubility product constant Ksp for Mg(OH)2: А. Ksp = [Mg2+]⋅[OH–] B. Ksp = [Mg2+]⋅[OH–]2 C. = aMg 2+ ⋅ a _ OH Ksp D. Ksp = 2[Mg2+]•2[OH–] E. Ksp = [Mg2+]•2[OH–] 8.9. The expression of the solubility product constant Ksp for Ca3 (PO4)2 is: 46 E. 8.14. The expression of the solubility product constant Ksp for CaF2: А. Ksp = [Ca2+] / [F–]2 B. Ksp = a(Ca2+) a(F–) C. Ksp = [Ca2+] + [F–] D. Ksp = [Ca2+][F–]2 E. Ksp = [Ca2+][2F–] O2 ⇄ 2SO3 is: А. [SO 2 ]2 Kp = B. C. 2- 8.16. The solubility of Ag2S (in mol/L) may be calculated according to the equation: А. Ksp S= 3 27 B. Ksp S= 3 4 C. Ksp S= 2 D. S= 3 4K [SO3 ]2 [SO2 ]2 ⋅ [O 2 ] Kp = [SO 2 ]2 ⋅ [O 2 ] [SO 3 ]2 Kp = 8.19. Point out, which of the given reactions should be held at the increased pressure according to Le Chatelier's principle. А. CO(gas) + Cl2(gas) = COCl2(gas) B. 3H2(gas) + N2(gas) = 2NH3(gas) C. N2(gas) + O2(gas) = NO2(gas) D. H2(gas) + Cl2(gas) = 2HCl(gas) E. Fe + H2O(gas) = FeO + H2(gas) sp E. S= K sp 8.17. The solubility of Fe(OH)3 (in mol/L) may be calculated according to the equation: А. Ksp S= 4 27 B. S= K 8.20. Which of the given statements is true for the system in the equilibrium state? А. The rates of the direct and reverse reactions are equal B. permanent losses of free Gibbs’ energy are needed C. the reactants concentrations are equal D. the reactants masses do not change sp S= 3 Kp = [SO3 ]2 ⋅ [O 2 ] [SO3 ]2 ⋅ [O 2 ] [SO 2 ]2 [SO3 ] E. Kp = [SO 2 ] ⋅ [O 2 ] D. 4 C. 4 8.18. Expression of the equilibrium constant for the reaction 2SO2 + 8.15. The expression of the solubility product constant Ksp for Ag2CrO4: А. Ksp = [Ag+]⋅[CrO42–] B. Ksp = [Ag+]2⋅[CrO42–] 2 C. (a ) ⋅ aCrO Ksp = Аg D. Ksp = [Ag+]+[Cr2O42–] E. Ksp = 2[Ag+]•[CO42–] + Ksp S= 3 Ksp 27 D. S= 3 4K sp 47 E. the permanent exchange of the substance and energy between the system and surroundings takes place Chapter 9. Chemical equilibrium D. 9.1. Select the true definition for the equilibrium constant through the given ones: А. the product of the reaction products equilibrium concentrations raised to the powers of their stoichiometric coefficients B. the product of the reactants equilibrium concentrations raised to the powers of their stoichiometric coefficients C. the rate constants of the reverse and direct reactions ratio D. the rate constants of the direct and reverse reactions ratio E. the product of the reactants concentrations changes E. [SO3 ]2 ⋅ [O 2 ] [SO 2 ]2 [SO 2 ]2 K eq = [SO3]2 ⋅ [O 2 ] K eq = 9.3. Select the true mathematical expression for the reaction FeO(solid) + H2(gas) ⇄ Fe(solid) + H2O(gas) equilibrium constant. А. [FeO] ⋅ [H 2 ] K eq = [Fe] ⋅ [H 2 O] B. K = [H 2 O] eq 9.2. Select the true mathematical expression for the reaction 2SO2 + O2 ⇄ 2SO3 equilibrium constant. А. [SO3 ]2 K eq = [SO 2 ]2 ⋅ [O 2 ] B. [SO 3 ] K eq = [SO 2 ] ⋅ [O 2 ] C. [SO 2 ]2 ⋅ [O 2 ] K eq = [SO3 ]2 [H 2 ] ⋅ [FeO] C. K eq = D. K eq = E. K eq = [H 2 O] [H 2 ] [H 2 ] [H 2O] [H 2 O] ⋅ [Fe] [H 2 ] ⋅ [FeO] 9.4. Methanol synthesis according to the equation of the reaction CO(g) + 2H2(g) ⇄ CH3OH(l) is the exothermal process. What variables and conditions should be changed for the product yield increasing? А. temperature increasing, pressure increasing 48 B. temperature increasing, methanol concentration increasing C. temperature decreasing, withdrawal of CO from the reaction area D. temperature increasing, withdrawal of hydrogen from the reaction area E. temperature decreasing, withdrawal of methanol from the reaction area C. temperature decreasing, nitrogen and hydrogen concentrations increasing D. the reacting mixture volume increasing, applying of a catalyst E. applying of a catalyst, temperature increasing 9.7. For which of the reactions given below the temperature and pressure increasing will shift the equilibrium toward the products formation: 9.5. What variables and conditions should be changed for the product SО3 yield increasing according to the reaction chemical equation 1. N2(gas) + 3H2(gas) ⇄ 2NH3(gas) Q; 2. C(solid) + O2(gas) ⇄ CO2(gas) + Q; SO2 + 1/2O2 ⇄ SO3 ; ∆H°298 = – 98.9 kJ/mol? А. temperature increasing, the reacting mixture volume decreasing B. temperature increasing, the reacting mixture volume increasing C. temperature decreasing, oxygen concentration decreasing D. temperature increasing, pressure decreasing E. temperature decreasing, pressure increasing 3. N2O4(liquid) ⇄ 2NO2(gas) – Q? А. 1 B. 2,3 C. для всіх наведених реакцій D. 1, 3 E. 2 9.8. The system state which does not change in time under the unchangeable variables and conditions, is called: А. equilibrium B. non-equilibrium C. isobaric D. stationary E. isothermal 9.6. What variables and conditions should be changed for the ammonium yield increasing according to the reaction chemical 9.9. For which of the given processes the temperature decreasing will cause the reaction rate increasing? А. endothermal B. exothermal C. isochoric D. adiabatic equation N2 + 3H2 ⇄ 2NH3 ; ∆H°298 = –92.4 kJ/mol? А. temperature decreasing, ammonium concentration increasing B. temperature increasing, pressure decreasing 49 E. isobaric 9.14. Will the equilibrium be shifted in the given reacting system under the temperature decreasing: N2 + 9.10. For which of the given reacting systems the pressure increasing will cause the equilibrium shifting towards direct reaction? А. H (g) + I (g) ⇄ 2HI(g) 2 3Н2 ⇄ 2NН3 + Q? А. – B. will shift right C. will not be shifted D. will shift left E. – 2 B. COCl (g) ⇄ CO(g) + Cl (g) 2 2 C. NH Cl(s) ⇄ HCl(g) + NH (g) 4 3 D. CO (g) + H (g) ⇄ CO(g) + 2 2 H2O(g) E. 2NO(g) + O (g) ⇄ 2NO (g) 2 9.15. Will the equilibrium be shifted in the given reacting system under the temperature decreasing: 2 СОCl2 ⇄ CO + Cl2 – Q? А. will shift left B. will not be shifted C. – D. will shift right E. – 9.11. For which of the given processes the temperature increasing will cause the product yield increasing? А. isobaric B. endothermal C. adiabatic D. isochoric E. exothermal 9.16. Will the equilibrium be shifted in the given reacting system under the hydrogen concentration 9.12. Will the equilibrium be shifted in the given reacting system under the temperature increasing: 2NO + increasing: Н2 + S ⇄ Н2S? А. – B. will shift right C. will not be shifted D. will shift left E. – O2 ⇄ 2NO2 + Q? А. – B. will shift left C. – D. will shift right E. will not be shifted 9.17. Will the equilibrium be shifted in the given reacting system under the hydrogen concentration 9.13. Will the equilibrium be shifted in the given reacting system under the temperature increasing: N2O4 increasing: Н2 + Cl2 ⇄ 2НCl? А. – B. will not be shifted C. – D. will shift right E. will shift left ⇄ 2NO2 – Q? А. – B. – C. will shift left D. will not be shifted E. will shift right 9.18. Will the equilibrium be shifted in the given reacting system under the pressure increasing: Н2 + І2 ⇄ 50 2НІ? А. will shift left B. – C. – D. will shift right E. will not be shifted D. will shift left E. – 9.23. Will the equilibrium be shifted in the given reacting system under the oxygen concentration decreasing: 3О2 ⇄ 2О3 + Q? А. – B. will shift left C. – D. will shift right E. will not be shifted 9.19. Will the equilibrium be shifted in the given reacting system under the pressure increasing: 2NO2 ⇄ 2NO + O2? А. – B. will not be shifted C. – D. will shift left E. will shift right 9.24. Will the equilibrium be shifted in the given reacting system under the temperature increasing: NH3 + H2O ⇄ NH4OH + Q? А. will not be shifted B. – C. – D. will shift left E. will shift right 9.20. Will the equilibrium be shifted in the given reacting system under the pressure increasing: N2 + 3H2 ⇄ 2NH3? А. will shift left B. – C. will shift right D. will not be shifted E. – 9.25. Will the equilibrium be shifted in the given reacting system under the pressure decreasing: NH3 + H2O ⇄ NH4OH + Q? А. will not be shifted B. will shift right C. – D. – E. will shift left 9.21. Will the equilibrium be shifted in the given reacting system under the temperature increasing: 3О2 ⇄ 2О3 + Q? А. – B. – C. will shift right D. will not be shifted E. will shift left 9.26. Will the equilibrium be shifted in the given reacting system under the ammonium concentration increasing: NH3 + H2O ⇄ NH4OH + Q? А. – B. will shift right C. will shift left D. will not be shifted E. – 9.22. Will the equilibrium be shifted in the given reacting system under the oxygen concentration increasing: 3О2 ⇄ 2О3 + Q? А. will shift right B. will not be shifted C. – 51 the equilibrium shifting right for the following reversible reaction 9.27. What variable change will cause the product yield increasing for the ethane hydration reaction: N2O4(г)⇄2 NO2(г), ∆H > 0 А. the reactant concentration decreasing B. the reaction product concentration increasing C. temperature increasing and pressure decreasing D. temperature increasing and pressure increasing E. temperature decreasing and pressure increasing С2Н4(g) + Н2⇄С2Н6(g) А. Н2 concentration decreasing B. reacting system volume increasing C. pressure decreasing D. pressure increasing E. С2Н4 concentration decreasing 9.28. What variable change will cause the equilibrium shifting left for the following reversible reaction 9.31. Chemical reactions which may occur in direct and reverse directions are called: А. catalytical B. kinetical C. thermochemical D. non-reversible E. reversible СО(g) + Н2О(g)⇄ СО2(g)+ Н2(g), ∆H < 0 А. temperature decreasing B. pressure increasing C. temperature increasing and pressure increasing D. temperature increasing E. reacting system volume change 9.32. What variable change will cause the equilibrium shifting right for the following reversible reaction 9.29. What variable change will cause the equilibrium shifting right for the following reversible reaction N2(g)+ О2(g)⇄2NО(g), ∆H > 0 А. temperature decreasing B. temperature increasing, the reaction product concentration decreasing C. nitrogen concentration decreasing D. pressure increasing E. oxygen concentration decreasing N2⇄2N, ∆H > 0 А. pressure increasing B. temperature increasing and pressure decreasing C. temperature decreasing D. the reactant concentration decreasing E. temperature decreasing and pressure increasing 9.30. What variable change will cause 52 Chapter 10. The concept of strong and weak electrolytes 10.1. Concentration of hydrogen-ion in a pure water is: А. 10–7 B. 10–10 C. 10–3 D. 10–14 E. 10–1 HNO3, NH4Cl, NH4OH, CH3COOH, CH3COON are weak ones: А. NH4Cl, NH4OH B. CH3COOH, CH3COONa C. AgCl, NH4Cl D. NH4OH, CH3COOH E. HCl, AgCl 10.2. Which of the listed correlations is true for the process of selfionization of water at 25 ºС? А. [H+]+[ОН-]=10-7mol/L B. [H+]+[ОН-]=10-14mol/L C. [H+] =[ОН-]=10-14mol/L D. [H+]/[ОН-]=10-7mol/L E. [H+]=[ОН-]=10-7mol/L 10.7. Which pair of ions cannot be found in a neutral solution? А. K+ and OH– B. Ca2+ and HCO– C. Na+ and SO42– D. H+ and CO32– E. Ag+ and NO3– 10.3. What is the concentration of [H+] in pure water at 25 ºС А. 10-1 B. 107 C. 10-14 D. 10-7 E. 1014 10.8. Choose the pair of electrolytes, which do not react in an aqueous solution? А. Fe(NO3)3 and NaOH B. NaBr and KOH C. K2CO3 and H2SO4 D. MgSO4 and (NH4)3PO4 E. Na2S and HCl 10.4. What is the concentration of [OH-] in pure water at 25 ºС А. 10-7 B. 107 C. 1014 D. 10-14 E. 10-1 10.9. Which reaction of the given ones is impossible: А. KCl + Br2 → KBr + Cl2. B. KI + Br2 → KBr + I2 C. NaI + Cl2 → NaCl + I2 D. KCl + І2 → KІ + Cl2 E. NaBr+ Cl2 → NaCl + Br2 10.5. Which of the following is the stronger acid? А. HClO2 B. HClO C. HClO3 D. HClO4 E. chlorous acid 10.10. Which base is weak electrolyte? А. NaOH B. Ca(OH)2 C. Mg(OH)2 D. Ba(OH)2 E. KOH 10.6. Which electrolytes HCl, AgCl, 10.11. Which acid is weak electrolyte? А. HCl 53 B. C. D. E. H2SO4 HBr H3BO3 HNO3 C. 5 D. 2 E. 3 10.16. Which particle of the given ones is an anion? А. Ca2+ B. K+ C. Fe3 D. NO3E. Na+ 10.12. Mistaken statement, that concern to iron (III) hydroxide: Fe(OH)3 – it is … А. strong electrolyte B. amphoteric hydroxide C. weak base D. insoluble in a water E. brown color of compound 10.17. Which compounds form ions of Mn2+ at the dissociation? А. MnCl2 B. MnO2 C. KMnO4 D. Na2MnO4 E. H2MnO4 10.13. Which one of the given molecular equation corresponds to the short ionic equation Сu2+ + S2– = CuS? А. CuCO3 + H2S = CuS + CO2 + H2O B. Cu(OH)2 + Na2S = CuS + 2NaOH C. Cu3(PO4)2 + 3(NH4)2S = 3CuS + 2(NH4)3PO4 D. CuBr2 + K2S = CuS + 2KBr E. CuCl2 + H2S = CuS + 2HCl 10.18. How many ions are forming at the dissociation of FeCl3? А. 10 B. 5 C. 6 D. 4 E. 4 10.14. Point out the row in which pH of compounds with the same molar concentration decrease: А. HCl, H2SO4, NH3, CH3COOH, NaOH B. CH3COOH, HCl, NaOH, NH3, H2SO4 C. NaOH, NH3, CH3COOH, HCl, H2SO4 D. H2SO4, HCl, NaOH, CH3COOH, NH3 E. NH3, CH3COOH, NaOH, HCl, H2SO4 10.19. Which of the following dissociation constant values of polyprotic acid is always larger? А. last B. first C. second D. the same E. third 10.20. Percent of dissociation NH4OH increase with: А. adding salt of ammonium B. concentrating of solution C. adding base D. dilution of solution E. cooling 10.15. How many ions are forming at the dissociation of (NH4)2SO4? А. 6 B. 4 10.21. Point out an aqueous solution 54 which has the worst electrical conductivity? Molarities of solutions are equal. А. HCN B. KCN C. KOH D. H2SO4 E. K2SO4 А. B. C. D. E. 10–7 10–3 10–9 10–5 10–11 10.27. pH equal zero in a solution of: А. 1M HCl B. 0.1M KOH C. 1M Ba(OH)2 D. 1M H2SO4 E. 0.1M HCl 10.22. Percent of dissociation of weak electrolytes increase with: А. increasing of concentration B. decreasing of acidity C. cooling D. increasing of acidity E. heating 10.28. Units of measurement of hydrogen-ion concentration in equation pH = -lg[H+] are: А. mol/cm3 B. mol/mL C. mol/L D. mol E. mol/кg 10.23. 0,1 М solution of which compound has the smallest concentration of ions? А. НCl B. H2SO4 C. NaNO3 D. СаCl2 E. СН3СООН 10.29. In the acidic solution the values of [H+] and рН are: А. [H+]< 10-7; рН< 7 B. [H+]>10-7; рН.> 7 C. [H+]>10-7; рН< 7 D. [H+]>10-7; рН= 7 E. [H+] = 10-7; рН = 7 10.24. In the basic medium рН and рОН are equal: А. рН < 7, рОН < 7 B. рН > 7, рОН >7 C. рН = 7, рОН = 7 D. рН > 7, рОН < 7 E. рН < 7, рОН >7 10.30. Which of the following pH values indicate an acidic solution at 25 ºС? А. 9,5 B. 1,2 C. 11,2 D. 10 E. 7,4 10.25. pH of 0,001 М solution of HCl equal: А. 0 B. 5 C. 7 D. 10 E. 3 10.31. Which of the following pH values indicate a basic solution at 25 ºС? А. 4,5 B. 7,3 C. 1,2 10.26. What is the concentration of hydroxide-ion in a solution, if рОН of its equal 9? 55 D. 4 E. 5,5 the stomach): А. 0,5 – 1,5 B. 0,9– 2,0 C. 1,5 – 3,0 D. 1,5 – 2,5 E. 2,5 – 3,5 10.32. In the acidic medium рН and рОН are equal: А. рН > 7, рОН >7 B. рН =7, рОН = 7 C. рН > 7, рОН<7 D. рН < 7, рОН<7 E. рН < 7, рОН >7 10.36. pH of the blood plasma is: А. 6,80 – 7,0 B. 7,35 – 7,45 C. 7,15 – 7,35 D. 8,01 – 8,25 E. 7,65 – 7,85 10.33. In the neutral medium рН and рОН are equal: А. рН =7, рОН = 7 B. рН > 7, рОН<7 C. рН < 7, рОН<7 D. рН < 7, рОН >7 E. рН > 7, рОН >7 10.37. What is the рН values range of the urine? А. 8,5 – 10,5 B. 2,0 – 3,5 C. 2,5 – 3,5 D. 7,5 – 9,5 E. 5,0 – 6,5 10.34. Which of the following pH values indicate a neutral solution at 25ºС? А. 9,5 B. 5 C. 7 D. 2,5 E. 13 10.38. Concentration of hydrogen-ion in a pure water is: А. 10–10 B. 10–1 C. 10–14 D. 10–3 E. 10–7 10.35. What is the pH of a sample of gastric juice (digestive juice in Chapter 11. Acids and bases theories. Self-ionization of water. pH E. рН = 7, рОН = 7 11.1. In the basic medium рН and рОН are equal: А. рН < 7, рОН < 7 B. рН > 7, рОН < 7 C. рН > 7, рОН >7 D. рН < 7, рОН >7 11.2. pH of 0,001 М solution of HCl equal: А. 10 B. 5 C. 0 56 D. 3 E. 7 values indicate a basic solution at 25 ºС? А. 4 B. 4,5 C. 7,3 D. 1,2 E. 5,5 11.3. What is the concentration of hydroxide-ion in a solution, if рОН of its equal 9? А. 10–7 B. 10–3 C. 10–9 D. 10–5 E. 10–11 11.9. In the acidic medium рН and рОН are equal: А. рН > 7, рОН >7 B. рН < 7, рОН<7 C. рН < 7, рОН >7 D. рН =7, рОН = 7 E. рН > 7, рОН<7 11.4. pH equal zero in a solution of: А. 0.1M HCl B. 1M H2SO4 C. 1M HCl D. 1M Ba(OH)2 E. 0.1M KOH 11.10. In the neutral medium рН and рОН are equal: А. рН > 7, рОН<7 B. рН =7, рОН = 7 C. рН > 7, рОН >7 D. рН < 7, рОН >7 E. рН < 7, рОН<7 11.5. Units of measurement of hydrogen-ion concentration in equation pH = -lg[H+] are: А. mol/кg B. mol/cm3 C. mol D. mol/L E. mol/mL 11.11. Which of the following pH values indicate a neutral solution at 25ºС? А. 13 B. 9,5 C. 7 D. 5 E. 2,5 11.6. In the acidic solution the values of [H+] and рН are: А. [H+]>10-7; рН.> 7 B. [H+]>10-7; рН= 7 C. [H+]< 10-7; рН< 7 D. [H+] = 10-7; рН = 7 E. [H+]>10-7; рН< 7 11.12. What is the pH of a sample of gastric juice (digestive juice in the stomach): А. 1,5 – 2,5 B. 1,5 – 3,0 C. 2,5 – 3,5 D. 0,9– 2,0 E. 0,5 – 1,5 11.7. Which of the following pH values indicate an acidic solution at 25 ºС? А. 9,5 B. 10 C. 1,2 D. 7,4 E. 11,2 11.13. pH of the blood plasma is: А. 6,80 – 7,0 B. 8,01 – 8,25 11.8. Which of the following pH 57 C. 7,35 – 7,45 D. 7,15 – 7,35 E. 7,65 – 7,85 Lowry concept of acids and bases, an base is the species which: А. accepts the electron B. C. donates the electron D. accepts the proton E. donates the proton 11.14. What is the рН values range of the urine? А. 2,5 – 3,5 B. 8,5 – 10,5 C. 7,5 – 9,5 D. 2,0 – 3,5 E. 5,0 – 6,5 11.19. Which of the given compounds do not belong to Lewis acid? А. AlCl3 B. С5H5N C. ZnCl2 D. FeCl3 E. no one through the listed compounds 11.15. Concentration of hydrogen-ion in a pure water is: А. 10–7 B. 10–10 C. 10–14 D. 10–3 E. 10–1 11.20. In acid-base reaction molecules of solvent: А. B. transfer electron from reducing agent to oxidizing agent C. transfer proton from acid to base D. transfer electron from oxidizing agent to reducing agent E. transfer proton from base to acid 11.16. According to the Bronsted-lowry concept of acids and bases amphoteric properties have the next electrolites: А. HClO, H2S, CH3NO2 B. HS-, NO2-, HSO4C. H2O, HCO3-,HPO42D. NH3, H3O+, HPO4E. H2O, HI, H3O+ 11.17. According to the BronstedLowry concept of acids and bases, an acid is the species which: А. accepts the proton B. donates a proton C. molecule contains a hydrogen-ion D. donates the electron E. has a free electron pair 11.21. Which of the given species is a Lewis base? А. CO2 B. Ca2+ C. F– D. CH4 E. H+ 11.18. According to the Bronsted- 58 Chapter 12. Protolytic processes А. transfer electron from reducing agent to oxidizing agent B. transfer electron from oxidizing agent to reducing agent C. D. transfer proton from base to acid E. transfer proton from acid to base 12.1. According to the Bronsted-lowry concept of acids and bases amphoteric properties have the next electrolites: А. H2O, HCO3-,HPO42B. HClO, H2S, CH3NO2 C. H2O, HI, H3O+ D. HS-, NO2-, HSO4E. NH3, H3O+, HPO412.2. According to the BronstedLowry concept of acids and bases, an acid is the species which: А. donates the electron B. has a free electron pair C. accepts the proton D. molecule contains a hydrogen-ion E. donates a proton 12.6. Which of the given species is a Lewis base? А. H+ B. F– C. CO2 D. CH4 E. Ca2+ 12.7. Which of the given species is a Lewis acid? А. BF3 B. NH3 C. SO42– D. CN– E. Cl– 12.3. According to the BronstedLowry concept of acids and bases, an base is the species which: А. accepts the proton B. donates the electron C. D. donates the proton E. accepts the electron 12.8. Which one of the aqueous solution of the given salts has an acidic pH? А. K2SO3 B. ZnSO4 C. Na3PO4 D. Na2B4O7 E. NaCl 12.4. Which of the given compounds do not belong to Lewis acid? А. ZnCl2 B. С5H5N C. FeCl3 D. AlCl3 E. no one through the listed compounds 12.9. Which one of the given salts cannot exist in an aqueous solution? А. FeCl3 B. K3PO4 12.5. In acid-base reaction molecules of solvent: 59 C. NH4Cl D. NaNO3 E. Cr2S3 А. B. C. D. E. 12.10. Color of litmus in a solution of iron (ІІІ) nitrate is: А. red B. pink C. violet D. blue E. colorless NaI Fe(NO3)3 BaCl2 K3PO4 K2CO3 12.16. Color of methyl orange in a solution of zinc sulfate is: А. yellow B. pink C. blue D. colorless E. orange 12.11. Select the salt, which does not hydrolyze: А. Al2(SO4)3 B. CrCl3 C. KNO2 D. KI E. FeSO4 12.17. Color of methyl orange in a solution of K2CO3 is: А. orange B. yellow C. red D. pink E. blue 12.12. Point out the salt which hydrolyze particularly: А. NaBr B. K2S C. Ca(NO3)2 D. BaSO4 E. Al2S3 12.18. Color of methyl orange in a solution of AlCl3 is: А. colorless B. yellow C. purple D. blue E. pink 12.13. Point out the salt which hydrolyze to the end: А. Al2(SO4)3 B. Na3PO4 C. Cr(NO3)3 D. Cr2S3 E. KNO3 12.19. Color of phenolphthalein in a solution of Na3PO4 is: А. colorless B. blue C. pink D. red E. orange 12.14. Which one of the given salts has a basic solution? А. Cr2(SO4)3 B. KCl C. NaNO3 D. AlCl3 E. Na2CO3 12.20. Color of methyl red in a solution of Cr2(SO4)3 is: А. yellow B. orange C. red D. blue E. colorless 12.15. Which one of the given salts has an acidic solution? 60 E. NH4Br 12.21. Select the salt, which do not hydrolyze: А. AlCl3 B. Ba(NO3)2 C. KNO2 D. ZnCl2 E. K2HPO4 12.26. Select the salt which increases the hydrogen-ion concentration, when dissolved in water? А. Na2CO3 B. K2SO4 C. ZnCl2 D. NaCl E. K3PO4 12.22. Point out the salt which can form as a product of hydrolysis a basic salt. А. KNO2 B. BaI2 C. AlCl3 D. Na2CO3 E. AgNO3 12.27. Select the salt, which do not hydrolyze: А. K2SO4 B. AlBr3 C. MnCl2 D. K2SO3 E. CuSO4 12.23. Complete and balance the equation of the hydrolysis of CaC2. What is the pH of the solution of CaC2 and what is the sum of the coefficients in the equation of hydrolysis? А. рН<7; 5 B. рН>7; 5 C. рН>7; 4 D. рН<7; 4 E. рН>7; 6 12.28. Point out the salt which hydrolyze in one step completely: А. Cr2S3 B. AlCl3 C. CuS D. no one through the listed salts E. Na2S 12.29. Which one from the given salts has a basic solution? А. Na2S B. CuCl2 C. FeCl3 D. KCl E. Na2SO4 12.24. Which one of the given salts forms as a product of hydrolysis a basic salt? А. Cr2(SO4)3 B. Na2SO4 C. AgNO3 D. CaCO3 E. K2CO3 12.30. What is the product of reaction between H2O and PCl5? А. HCl + H3PO3 B. HCl + H3PO4 C. Cl2 + H3PO4 D. P2O3 + HCl E. P2O5 + HCl 12.25. Which one of the given salts forms a neutral solution? А. NH4Cl B. NH4NO3 C. (NH4)2SO4 D. CH3COONH4 12.31. Select the salt, which hydrolyze 61 in an aqueous solution: А. KI B. LiBr C. NaCl D. KI E. NaF 12.36. Point out the salt which hydrolyze to the end: А. CuSO4 B. Zn(NO3)2 C. K2S D. Na2CO3 E. Аl2S3 12.32. Which one of the given salts reacts with an acid and forms as a product of reaction a gas? А. Na2CO3 B. CuSO4 C. Na2SiO3 D. K2SO4 E. Ca3(PO4)2 12.37. Which ions can be at the same time in a solution? А. Fe3+, OH– B. Fe3+ , Cl– C. Ba2+, SO42– D. Fe2+, OH– E. Ag+, Cl– 12.33. Some medicine can destroy in acidic medium. Which one of the given salt is incompatible with the medicine in an aqueous solution? А. NaHCO3 B. K3PO4 C. KI D. ZnSO4 E. NaCl 12.38. The expression of the constant of hydrolysis Кh = Кw/Kb is for a salt of: А. CH3COONa B. CuSO4 C. (NH4)2S D. KCN E. NaCl 12.39. The expression of the constant of hydrolysis Кh = Кw/(Ка · Kb) is for a salt of: А. (NH4)2S B. NH4Cl C. Li2S D. NaCN E. Fe(NO3)3 12.34. Some antibiotics can destroy in acidic medium. Which one of the given salt is incompatible with the antibiotics in an aqueous solution? А. Na3PO4 B. CaCl2 C. NH4Cl D. Na2CO3 E. KI 12.40. The expression of the constant of hydrolysis Кh = Kw/(Ka· Kb) is for a salt of: А. CH3COONa B. NH4Cl C. К3PO4 D. CH3COONH4 E. FeCl3 12.35. Select the salt, which hydrolyze in an aqueous solution: А. Sr(NO3)2 B. KNO3 C. NaNO3 D. Ba(NO3)2 E. NH4NO3 12.41. The expression of the constant of hydrolysis Кh = Кw/Kb is for a 62 salt of: А. Na2CO3 B. K2SO4 C. K3PO4 D. NH4Cl E. LiNO3 А. B. C. D. E. H2O H2SO4 BaSO4 KOH Na2CO3 12.44. The direction of protolytical reaction СН3СООН + С6Н5ОNa → ← C6H5OH + CH3COONa is shifted: А. reaction is impossible B. dynamic equilibrium C. forward D. E. reverse 12.42. The expression of the constant of hydrolysis Кh = Кw/Ka is for a salt of: А. NaCN B. (NH4)3PO4 C. Na2SO4 D. CH3COONH4 E. (NH4)2SO4 12.43. Percent of hydrolysis of CuSO4 decrease with adding solution of: Chapter 13. Reactions with electrons transferring reaction with an iron sulfate in acidic medium find out properties of: А. B. oxidizing agent C. does not show the redox properties D. oxidizing agent and reducing agent E. reducing agent 13.1. How will change the redox properties in a row: Cl– – Br– – I– А. does not change B. C. increased D. decreases E. a bromine is a stronger reduction agent, than chlorine 13.2. What is the oxidation number of a molybdenum in the compound (NH4)6Mo7O24 ? А. +3 B. +2 C. +5 D. +1 E. +6 13.4. Mercury has the oxidation number +2 in the following compound? А. Hg2SO4 B. Hg2(NO3)2•2H2O C. Hg2O D. Hg2Cl2 E. K2[HgI4] 13.3. Potassium permanganate in a 63 + H2O 13.5. What is the oxidation number of a Arsenic in the compound AsH3? А. +3 B. –3 C. +5 D. –5 E. +1 13.9. What is the oxidation state of the central ion in the coordination compound [Cr(H2O)4Cl2]Cl. А. +4 B. 0 C. +2 D. +3 E. +6 13.6. Nitrogen(ІІ) oxide has redox properties. What properties it finds out in the reaction NO+ KMnO4 + H2SO4 → ? А. B. oxidizing properties C. oxidizing and reducing properties D. does not change the oxidation number E. reducing properties 13.10. How many electrons gains the potassium dichromate.K2Cr2O7. What is the oxidation number of a Chromium when it reduced in the acidic medium? А. 3, +3 B. 3, +4 C. 8, +2 D. 6, +3 E. 4, +2 13.7. Which reaction through the listed ones correspondsto the intermolecular oxidationreduction reaction. А. HCl + CrO3 = CrCl3 + Cl2 + H2O B. Cl2 + H2O = HCl + HClO C. 2CuCl +Cl2 = 2CuCl2 D. KMnO4 + HCl = MnCl2 + Cl2 + KCl + H2O E. Au2O3 = Au + O2 13.11. Which compound through the listed ones has both oxidizing and reducing properties? А. CrO3 B. PbO2 C. CO2 D. SO3 E. SO2 13.12. Which equation through the listed ones corresponds to the disproportionation? А. I2O5 + H2O = 2HIO3 B. Cl2O7 + H2O = 2HClO4 C. Cl2O + H2O = 2HClO D. Br2O + H2O = 2HBrO E. ClO2 + H2O = HClO2 + HClO3 13.8. Which reaction through the listed ones correspondsto the disproportionation oxidationreduction reaction. А. Au2O3 → Au + O2 B. MnO2 + HCl → MnCl2 + Cl2 + H2O C. S + KOH → K2SO3 + K2S + H2O D. FeCl2 + Cl2 → FeCl3 E. HCl + Cr2O3 → CrCl3 + Cl2 13.13. Which compound through the listed ones has both oxidizing and reducing properties? : А. NO2 64 B. N2O5 C. NH3 D. HNO3 E. NH4Cl 13.18. Which reaction through the listed ones is impossible: А. NaI + Cl2 → NaCl + I2 B. KCl + І2 → KІ + Cl2 C. NaBr+ Cl2 → NaCl + Br2 D. KCl + Br2 → KBr + Cl2 E. KI + Br2 → KBr + I2 13.14. Which equation through the given ones corresponds to the the reaction of disproportionation? А. MnO2 + HCl → MnCl2 + Cl2 + H2O B. FeCl2 + Cl2 → FeCl3 C. S + KOH → K2SO3 + K2S + H2O D. Au2O3 → Au + O2 E. HCl + Cr2O3 → CrCl3 + Cl2 + H2O 13.19. In which of the given compounds iron can show reducing properties only? А. Fe2O3 B. FeCl2 C. K2FeO4 D. Fe E. FeCl3 13.15. NaNO2 has reducing properties when rescts with: А. KMnO4 B. NaHCO3 C. KI D. NH3 E. H2S 13.20. In which of the given compounds iron can show oxidizing properties only? А. FeCl2 B. Fe2O3 C. K2FeO4 D. Fe E. FeCl3 13.16. What is the oxidation state of the central ion in the coordination compound Na2[Fe(CN)5NO] ? А. +1 B. +3 C. +6 D. 0 E. +2 13.21. In which of the given compounds iron can show both oxidizing and reducing properties? 1. K2FeO4 2. Fe 3. FeCl2 4. FeCl3 5. Fe2O3 Give an answer as the sum of numbers of these compounds. А. 12 B. 10 C. 9 D. 11 E. 13 13.17. The transformation Cr+3 → CrO42– is: А. the process of oxidation in basic solution B. the process of reduction in acidic solution C. the process of reduction in basic solution D. E. the process of oxidation in acidic solution 13.22. In which of the given compounds iron can show both oxidizing and reducing properties? 1. K2FeO4 2. Fe 3. FeO 4. FeCl3 5. Fe2O3 Give an 65 answer as the sum of numbers of these compounds. А. 13 B. 10 C. 9 D. 11 E. 12 C. CrCl3 D. K2CrO4 E. Cr 13.27. In which of the given compounds Chromium can show oxidizing properties only? А. CrCl2 B. K2CrO4 C. Cr2O3 D. CrCl3 E. Cr 13.23. In which of the given compounds Manganese can show reducing properties only? А. Mn B. K2MnO4 C. KMnO4 D. Mn2O7 E. MnO2 13.28. In which of the given compounds Chromium can show oxidizing properties only? А. Cr2O3 B. CrCl3 C. K2Cr2O7 D. Cr E. CrCl2 13.24. In which of the given compounds Manganese can show oxidizing properties only? А. K2MnO4 B. MnO2 C. Mn D. Mn2O3 E. KMnO4 13.29. In which from the stated below matters Chromium can show oxidizing properties only? А. Cr2O3 B. Cr C. CrO D. CrCl3 E. CrO6 13.25. In which of the given compounds Manganese can show both oxidizing and reducing properties? 1. KMnO4 2. K2MnO4 3. Mn 4. MnO2 5. Mn2O7 Give an answer as the sum of numbers of these compounds. А. 6 B. 4 C. 7 D. 8 E. 5 13.30. In which of the given compounds Chromium can show both oxidizing and reducing properties? 1. K2CrO4 2. CrCl3 3. Cr 4. Cr2O3 5. CrO Give an answer as the sum of numbers of these compounds. А. 9 B. 10 C. 8 D. 11 E. 7 13.26. In which of the given compounds Chromium can show reducing properties only? А. CrCl2 B. K2Cr2O7 13.31. In which of the given 66 compounds Chromium can show both oxidizing and reducing properties? 1. K2CrO4 2. CrCl3 3. Cr 4. K2Cr2O7 5. CrO Give an answer as the sum of numbers of these compounds. А. 8 B. 10 C. 11 D. 9 E. 7 oxidizing properties only? А. CoSO4 B. CoCl2 C. [Co(NH3)6]Cl3 D. CoO E. Co 13.36. In which of the given compounds Cobalt can show both oxidizing and reducing properties? 1. CoO 2. CoCl3 3. Co 4. [Co(NH3)6]Cl3 5. CoCl2 Give an answer as the sum of numbers of these compounds. А. 7 B. 9 C. 4 D. 5 E. 6 13.32. In which of the given compounds Chromium can show both oxidizing and reducing properties? 1. CrO3 2. CrCl3 3. Cr 4. K2Cr2O7 5. CrCl2 Give an answer as the sum of numbers of these compounds. А. 11 B. 7 C. 9 D. 10 E. 8 13.37. In which of the given compounds Cobalt can show both oxidizing and reducing properties? 1. CoO 2. CoCl3 3. Co 4. [Co(NH3)6]Cl3 5. CoSO4 Give an answer as the sum of numbers of these compounds. А. 6 B. 7 C. 5 D. 9 E. 4 13.33. In which of the given compounds Cobalt can show reducing properties only? А. CoCl3 B. [Co(NH3)6]Cl3 C. CoO D. Co E. CoCl2 13.38. In which of the given compounds Copper can show reducing properties only? А. CuO B. CuCl2 C. Cu2O D. CuCl E. Cu 13.34. In which of the given compounds Cobalt can show oxidizing properties only? А. Co B. CoO C. CoCl2 D. CoSO4 E. CoCl3 13.39. In which of the given compounds Copper can show oxidizing properties only? 13.35. In which of the given compounds Cobalt can show 67 А. CuCl2 B. CuCl C. Cu D. Cu2O E. K[Cu(CN)2] D. 3 E. 1 13.44. What is the number of electrons, losted or gained according with the transformation: NO→ NO2. А. 2 B. 5 C. 1 D. 3 E. 4 13.40. In which of the given compounds Copper can show both oxidizing and reducing properties? 1. K[Cu(CN)2] 2. CuCl2 3. Cu 4. [Co(NH3)6]Cl3 5. CuCl Give an answer the sum of numbers of these matters. А. 6 B. 7 C. 5 D. 4 E. 8 13.45. What is the number of electrons, losted or gained according with the transformation: MgO→ Mg0 . А. 1 B. 2 C. 5 D. 4 E. 3 13.41. What is the number of electrons, losted or gained according with the transformation: HNO3 → NO2. А. 3 B. 4 C. 2 D. 5 E. 1 13.46. What is the number of electrons, losted or gained according with the transformation: HNO3 → NO. А. 5 B. 3 C. 1 D. 2 E. 4 13.42. What is the number of electrons, losted or gained according with the transformation: H2SO3 → H2SO4 . А. 4 B. 1 C. 2 D. 5 E. 3 13.47. What is the number of electrons, losted or gained according with the transformation: N2 → NO. А. 5 B. 1 C. 4 D. 3 E. 2 13.43. What is the number of electrons, losted or gained according with the transformation: S0 → H2S. А. 4 B. 5 C. 2 13.48. What is the number of electrons, losted or gained according with the transformation: O2 → CuO. А. 1 68 B. 4 C. 2 D. 5 E. 3 13.53. What is the number of electrons, losted or gained according with the transformation: HNO3 → NH4Cl. А. 7 B. 6 C. 9 D. 8 E. 5 13.49. What is the number of electrons, losted or gained according with the transformation: H2SO4 → S0. А. 4 B. 7 C. 5 D. 8 E. 6 13.54. What is the number of electrons, losted or gained according with the transformation: HNO3 → NH3. А. 5 B. 7 C. 8 D. 6 E. 9 13.50. What is the number of electrons, losted or gained according with the transformation: S0 → H2SO4 . А. 8 B. 6 C. 5 D. 4 E. 7 13.55. What is the number of electrons, losted or gained according with the transformation: HNO3 → N2O А. 8 B. 6 C. 4 D. 5 E. 7 13.51. What is the number of electrons, losted or gained according with the transformation: H2S → SO2 . А. 7 B. 4 C. 5 D. 6 E. 3 13.56. What is the number of electrons, losted or gained according with the transformation: H2SO4 → H2S. А. 5 B. 6 C. 7 D. 4 E. 8 13.52. What is the number of electrons, losted or gained according with the transformation: NH3 → N2. А. 7 B. 3 C. 5 D. 6 E. 4 69 Chapter 14. Experimental studying of reduction-oxidation reactions the transformation: MgO→ Mg0 . А. 4 B. 5 C. 3 D. 2 E. 1 14.1. What is the number of electrons, losted or gained according with the transformation: HNO3 → NO2. А. 5 B. 2 C. 4 D. 3 E. 1 14.6. What is the number of electrons, losted or gained according with the transformation: HNO3 → NO. А. 1 B. 2 C. 4 D. 5 E. 3 14.2. What is the number of electrons, losted or gained according with the transformation: H2SO3 → H2SO4 . А. 5 B. 3 C. 4 D. 2 E. 1 14.7. What is the number of electrons, losted or gained according with the transformation: N2 → NO. А. 1 B. 2 C. 4 D. 5 E. 3 14.3. What is the number of electrons, losted or gained according with the transformation: S0 → H2S. А. 5 B. 4 C. 3 D. 2 E. 1 14.8. What is the number of electrons, losted or gained according with the transformation: O2 → CuO. А. 3 B. 4 C. 5 D. 1 E. 2 14.4. What is the number of electrons, losted or gained according with the transformation: NO→ NO2. А. 2 B. 5 C. 4 D. 3 E. 1 14.9. What is the number of electrons, losted or gained according with the transformation: H2SO4 → S0. А. 5 B. 7 14.5. What is the number of electrons, losted or gained according with 70 C. 6 D. 8 E. 4 B. C. D. E. 14.10. What is the number of electrons, losted or gained according with the transformation: S0 → H2SO4 . А. 6 B. 5 C. 4 D. 8 E. 7 6 7 5 9 14.14. What is the number of electrons, losted or gained according with the transformation: HNO3 → NH3. А. 8 B. 7 C. 5 D. 6 E. 9 14.11. What is the number of electrons, losted or gained according with the transformation: H2S → SO2 . А. 4 B. 3 C. 7 D. 6 E. 5 14.15. What is the number of electrons, losted or gained according with the transformation: HNO3 → N2O А. 4 B. 7 C. 6 D. 8 E. 5 14.12. What is the number of electrons, losted or gained according with the transformation: NH3 → N2. А. 3 B. 5 C. 4 D. 6 E. 7 14.16. What is the number of electrons, losted or gained according with the transformation: H2SO4 → H2S. А. 7 B. 5 C. 8 D. 6 E. 4 14.13. What is the number of electrons, losted or gained according with the transformation: HNO3 → NH4Cl. А. 8 71 Chapter 15. Coordination compounds. Reactions of coordination compounds formation 15.1. The most stable coordination compounds exist for: А. transition metals B. alkali metals C. alkaline earth metals D. lantanoids E. s-elements 15.5. Select the anion complex ion from the given ones: А. [Cr(H2O)3(CN)3] B. [Zn(OH)4] C. [Cu(NH3)4] D. [Cr(H2O)3Br] E. [Ag(NH3)2] 15.2. Point out the formula of the coordination compound for which the empirical formula is PtCl4·4NH3 and the coordination number of Pt(IV) is 6. А. [Pt(NH3)2Cl4] B. [Pt(NH3)2Cl2]Cl2 C. [PtCl4] D. [Pt(NH3)4Cl2]Cl2 E. [PtCl3(NH3)3]Cl 15.6. Select the cation complex ion from the given ones: А. [Zn(OH)4] B. [Cr(H2O)4(CN)2] C. [Ag(СN)2] D. [Cu(СNS)4] E. [Al(OH)6] 15.7. Select the anion complex ion from the given ones: А. [Cr(NH3)3(CN)3] B. [Cu(H2O)4] C. [Al(OH)6] D. [Cu(NH3)4] E. [Cu(NH3)2Cl2] 15.3. Point out the formula of the coordination compound for which the empirical formula is PtCl4·6NH3 and the coordination number of Pt(IV) is 6. А. К2[PtCl6] B. [Pt(NH3)6]Cl4 C. [PtCl3(NH3)3]Cl D. [Pt(NH3)4Cl2]Cl2 E. [Pt(NH3)2Cl4] 15.8. Select the cation complex ion from the given ones: А. [HgI4] B. [Cr(NH3)4(CNS)2] C. [Zn(OH)4] D. [Cu(СN)4] E. [Cu(H2O)(CN)3] 15.4. Point out the formula of the coordination compound for which the empirical formula is 2NH4Cl· PtCl4? А. [Pt(NH3)4Cl2]Cl2 B. [Pt(NH3)6]Cl4 C. (NH4)2[PtCl6] D. [Pt(NH3)2Cl4] E. [Pt(NH3)2Cl3]Cl 15.9. What are the oxidation states of the central metal ions in the complex ions [Fe(CN)6]4– and [Cr(H2O)4F2]+ ? А. +2, +2 B. +4, +3 C. +2, +3 D. +3, +2 72 E. +3, +3 15.14. Select the most stable complex ion of mercury through the listed ones, if the complex ions have the given values of the constants of dissociation: А. [Hg(СN)4]2– Кd = 3·10–42 B. [Hg(CNS)4]2– Кd = 1,3·10–22 C. [HgI4]2– Кd = 1,38·10–30 D. [HgBr4]2– Кd = 1·10–21) E. [HgCl4]2– Кd = 6·10–16 15.10. What are the oxidation states of the central metal ions in the coordination compounds [Cu(NH3)4]SO4 and K2[Pt(OH)2Cl4]? А. +1, +2 B. +2, +3 C. +2, +4 D. +2, +2 E. +1, +4 15.15. Select the least stable complex ion of zinc through the listed ones, if the complex ions have the given values of the constants of dissociation: А. [Zn(OH)4]2– Кd = 2,2·10–15 B. [Zn(CNS)4]2– Кd = 5·10–2 C. [Zn(CN)4]2– Кd = 1,0·10–10 D. [Zn(NH3)4]2+ Кd = 2,0·10–9 E. [ZnЕДТА]2– Кd = 3,2·10–17 15.11. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound Na3[Cr(OH)6]? А. +3, –4 B. +3, –2 C. +3, –3 D. +3, +3 E. +2, +4 15.16. Select the most stable complex ion of Ag through the listed ones, if the complex ions have the given values of the constants of dissociation: А. [Ag(NO2)2]– Кd = 1,8·10–3 B. [Ag(S2O3)2]3– Кd = 3,5·10–14 C. [Ag(CN)2]– Кd = 1,4·10–20 D. [AgCl2]– Кd = 9,1·10–6 E. [Ag(NH3)2]+ Кd = 5,8·10–8 15.12. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound [Co(NH3)4(CNS)2]Cl ? А. +3, 6 B. +2, 4 C. +3, 4 D. +3, 2 E. +2, 6 15.17. The expression of the constants of dissociation of complex ion for the compound K4[Fe(CN)6] is: А. [Fe 2 + ] ⋅ 6[CN − ] [[Fe(CN) 6 ] 4− ] B. [ Fe 2 + ] + [CN − ] 6 15.13. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound K2[Pt(OH)2Cl4] ? А. +2, –4 B. +2, –2 C. +4, +2 D. +4, 0 E. +4, –2 [[Fe(CN) 6 ] 4 − ] C. [Fe 2+ ] ⋅ [CN − ]6 [[Fe(CN) 6 ] 4 − ] 73 А. [Hg 2+ ] ⋅ [I − ] 4 D. [Fe 3+ ] ⋅ [CN − ]6 [[HgI 4 ] 2− ] 3− [[Fe(CN ) 6 ] ] B. [K + ] 2 ⋅ [Hg 2+ ] ⋅ [I − ] 4 E. [ K + ] 4 ⋅ [[Fe(CN ) 6 ] 4− ] [ K 2 [ HgI 4 ]] [K 4 [Fe (CN ) 6 ]] 2+ 2 15.18. The expression of the constants of dissociation of complex ion for the compound [Cu(NH3)4]SO4 is: А. [Cu 2+ ] ⋅ [ NH 3 ] 4 2+ C. [Hg ] ⋅ [I − ] [[HgI 4 ]2 − ] D. [ K + ] 2 ⋅ [[HgI 4 ] 2 − ] [ K 2 [HgI 4 ]] E. [Hg 2+ ] ⋅ 4[I − ] [[Cu ( NH 3 ) 4 ] ] B. [Cu 2+ ] 2 ⋅ [ NH 3 ] 4 [[Cu ( NH 3 ) 4 ] 2+ ] C. [Cu 2+ ] ⋅ 4[ NH 3 ] [[HgI 4 ] 2− ] 15.21. The expression of the constants of dissociation of complex ion for the compound Na2[Zn(OH)4] is: А. [ Na + ] 2 ⋅ [ Zn 2+ ] ⋅ [OH − ] 4 ] [[Cu ( NH 3 ) 4 ] 2+ ] D. [Cu 2+ ] + [ NH 3 ] 4 [ Na 2 [ Zn(OH) 4 ]] [[Cu ( NH 3 ) 4 ] 2+ ] + 2 B. [ Na ] ⋅ [ Zn(OH) 4 ] 2− ] E. [[Cu ( NH 3 ) 4 ] 2+ ] ⋅ [SO 24− ] [ Na 2 [ Zn(OH) 4 ]] [[Cu ( NH 3 ) 4 ]SO 4 ] 15.19. The expression of the constants of dissociation of complex ion for the compound [Co(NH3)6]Cl2 is: А. [Co 2+ ] ⋅ [ NH 3 ] 6 [[Co( NH 3 ) 6 ] 2+ ] C. [ Zn 2 + ] + [OH − ] 4 [[Zn(OH) 4 ] 2− ] D. [ Zn 2+ ] ⋅ 4[OH − ] [[Zn(OH) 4 ] 2− ] E. [Zn 2+ ] ⋅ [OH − ] 4 [[Zn(OH) 4 ] 2− ] B. [Co 2 + ] + [ NH ] 6 3 [[Co( NH 3 ) 6 ] 2 + ] C. [Co( NH 3 ) 6 ] ⋅ [Cl − ] 2 [[Co( NH 3 ) 6 ]Cl 2 ] D. [Co 2+ ] ⋅ 6[ NH ] 3 [[Co( NH 3 ) 6 ] 2+ ] E. [Co 2 + ] ⋅ 6[ NH 3 ] ⋅ 2[Cl − ] [[Co ( NH 3 ) 6 ]Cl 2 ] 15.20. The expression of the constants of dissociation of complex ion for the compound K2[HgI4] is: 74 15.22. Select the most stable amminecomplex through the listed ones, if the complex ions have the given values of the constants of dissociation: А. [Co(NH3)6]3+ Кd = 6,2·10–36 B. [Cu(NH3)4]2+ Кd = 9,3·10–13 C. [Ni(NH3)6]2+ Кd = 1,2·10–8 D. [Zn(NH3)4]2+ Кd = 2,0·10–9 E. [Hg(NH3)4]2+ Кd = 5,2·10–20 15.23. The quantitative characteristic of complex ions stability in solution is: А. the equilibrium constant B. the constant of the complex formation C. the constant of hydrolysis D. the percent of dissociation E. the constant of dissociation D. 10 E. 6 15.28. Complete and balance the equation of the reaction AgBr + Na2S2O3 → Point out the sum of the coefficients of this equation. А. 5 B. 4 C. 8 D. 6 E. 7 15.24. What is the product of the reaction of interaction of copper sulfate with the excess of ammonium hydroxide? А. [Cu(NH3)4](OH)2 B. [Cu(NH3)2(H2O)2]SO4 C. Cu(OH)2 D. [Cu(OH)2(NH3)2] E. (NH4)2[Cu(OH)4] 15.25. What is the product of the reaction of Cu(OH)2 dissolving in the excess of concentrated КОН? А. [Cu(H2O)4](OH)2 B. reaction doesn’t pass C. [Cu(OH)2(H2O)2] D. K2[Cu(OH)4] E. K[Cu(OH)3] 15.26. Which coordination compound through the listed ones will form when the solution of HgCl2 would be acted with the excess of KI? А. K2[HgI2Cl2] B. [Hg(H2O)4]Cl2 C. K2[HgCl4] D. K2[HgI4] E. [HgI2] 15.29. Complete and balance the equation of the reaction [Cu(H2O)4]SO4 + NH3 → Point out the sum of the coefficients of this equation. А. 11 B. 9 C. 12 D. 8 E. 10 15.30. What coordination number is the most common for Fe2+? А. 6 B. 4 C. 8 D. 2 E. 5 15.31. The most stable coordination compounds with aminocarbon acids can form the following ions: А. Mn, Mg, Zn B. Mn, Ni, Fe C. Co, Fe, Zn D. Cu, Zn, Co E. Fe, Mn, Mg 15.27. Complete and balance the equation of the reaction Cu(OH)2 + NH4OHconc. → Point out the sum of the coefficients of this equation. А. 9 B. 13 C. 8 15.32. What is the oxidation state of the central metal ion in the coordination compound 75 Na2[Fe(CN)5NO]? А. +6 B. +1 C. +2 D. +3 E. 0 D. 16 E. 4 15.37. Which ligands through the listed ones are monodentate? А. OH– and SO42– B. Cl– and P2O74– C. NH3 and SO42– D. NH3 and CN– E. Н2O and CO32– 15.33. Zinc can dissolve in the excess of an alkali solution owing to the complex ion formation: А. [Zn(OH)4]2– B. [Zn(H2O)4]2+ C. [ZnCl4]2– D. [Zn(NH3)4]2+ E. ZnO22– 15.38. Which ligands through the listed ones are bidentate? А. Н2O and CNS– B. Н2O and Br– C. Cl– and CN– D. CO32– and P2O74– E. NH3 and CN– 15.34. What is the geometry of the hybrid orbitals in the coordination compounds with the coordination number 2 of the central metal ion like [Ag(NH3)2]+? А. square pyramid B. linear C. Tetrahedral D. square planar E. octahedral 15.39. Which ligands through the listed ones are monodentate? А. NH3, CO32–, CNS– B. CO, NO, SO42– C. CO32–, Cl–, OH– D. NH3, CO, CN– E. С2О42–, CNS–, CN– 15.40. The components of coordination compounds are: А. the central metal ion and positively or negatively charged ions B. the central metal ion and the complex cation C. the complex ion and the external coordination sphere D. the central metal ion and the complex anion E. the central metal ion and ligands 15.35. What will be the product of the reaction between ZnCl2 and potassium hexacyanoferrate (II)? А. K2[Zn(CN)4] B. Fe[Zn(CN)4] C. Zn2[Fe(CNS)6] D. Zn2[Fe(CN)6] E. Zn3[Fe(CN)6]2 15.36. Complete and balance the equation of the reaction K4[Fe(CN)6] + Cl2 → Point out the sum of the coefficients of this equation. А. 6 B. 8 C. 7 15.41. What kind of chemical bonding is obligatory in coordination compounds? А. ionic bonding 76 B. metallic bonding C. covalent bonding D. donor-acceptor bonding E. hydrogen bonding А. [Ni(OH)2(NH3)2](NO3)2 B. [Ni(H2O)2(NH3)2]NO2 C. [Ni(H2O)2(NH3)2]NO3 D. [Ni(H2O)2(NH3)2](NO3)2 E. [Ni(H2O)2(NO3)2] 15.42. What is the molecular formula of the coordination compound diammineditiocyanocopper(ІІ)? А. (NH4)2[Cu (CN)2] B. (NH4)2[Cu (CNS)4] C. [Cu(NH3)2(CN)2]S D. [Cu(NH3)2](CNS)2 E. [Cu(NH3)2(CNS)2] 15.45. What is the molecular formula of the coordination compound potassium dihydroxotetrachlorochromate(ІІІ ) А. [KCrCl4](OH)2 B. K[Cr(H2O)2Cl4] C. K[Cr(OH)2Cl2] D. K3[Cr(H2O)2(OH)2Cl2] E. K3[Cr(OH)2Cl4] 15.43. What is the molecular formula of the coordination compound pentaamminebromocobalt(ІІІ) sulfate? А. [Co(NО2)5Br]SO4 B. [Co(NH3)5Br]SO4 C. [Co(NH4)5Br](SO4)2 D. [Co(NH3)5Br]SO3 E. [Co(NH3)5SO4]Br 15.46. What is the molecular formula of the coordination compound dichloroaquatriamminecobalt(ІІІ) bromide? А. [Co(H2O)BrCl2(NH3)2] B. [Co(H2O)Br 2(NH3)3]Cl C. [Co(H2O)Cl2(NH3)3]Br D. [CoCl2(NH3)3H2O]Br2 E. [Co(NH3)3(H2O)Br]Cl2 15.44. What is the molecular formula of the coordination compound diaquadiamminenicol(ІІ) nitrateт? Chapter 16. Experimental studying of coordination compounds properties 16.1. The expression of the constants of dissociation of complex ion for the compound K4[Fe(CN)6] is: А. [Fe 2 + ] ⋅ [CN − ] 6 [[Fe(CN) 6 ] 4− ] B. [K + ] 4 ⋅ [[Fe(CN) 6 ] 4 − ] [K 4 [ Fe(CN) 6 ]] C. [Fe 2+ ] + [CN − ]6 [[Fe(CN) 6 ] 4 − ] D. [Fe 3+ ] ⋅ [CN − ] 6 [[Fe(CN ) 6 ]3− ] 77 D. [Hg 2 + ]2 ⋅ [I − ] E. [Fe 2+ ] ⋅ 6[CN − ] [[HgI 4 ]2 − ] 4− [[Fe(CN ) 6 ] ] E. [K + ] 2 ⋅ [[HgI 4 ] 2− ] 16.2. The expression of the constants of dissociation of complex ion for the compound [Cu(NH3)4]SO4 is: А. [Cu 2+ ] ⋅ [ NH 3 ] 4 [[Cu ( NH 3 ) 4 ] 2 + ] B. [Cu 2+ ] + [ NH 3 ] 4 B. [ Na + ] 2 ⋅ [ Zn 2+ ] ⋅ [OH − ] 4 ] C. [Cu 2+ ] 2 ⋅ [ NH 3 ]4 [ Na 2 [ Zn (OH) 4 ]] [[Cu ( NH 3 ) 4 ] 2+ ] C. [ Zn 2+ ] ⋅ 4[OH − ] D. [Cu 2+ ] ⋅ 4[ NH 3 ] [[Zn(OH) 4 ] 2− ] [[Cu ( NH 3 ) 4 ] 2+ ] D. [ Na + ] 2 ⋅ [ Zn(OH) 4 ] 2− ] E. [[Cu ( NH 3 ) 4 ] 2 + ] ⋅ [SO 24 − ] [ Na 2 [ Zn (OH) 4 ]] [[Cu ( NH 3 ) 4 ]SO 4 ] E. 16.3. The expression of the constants of dissociation of complex ion for the compound [Co(NH3)6]Cl2 is: А. [Co 2+ ] ⋅ 6[ NH 3 ] [[Co ( NH 3 ) 6 ] 2 + ] B. [Co 2 + ] ⋅ 6[ NH3 ] ⋅ 2[Cl− ] [[Co( NH3 )6 ]Cl 2 ] C. [Co 2+ ] ⋅ [ NH 3 ]6 [[Co ( NH 3 ) 6 ] 2 + ] D. [Co( NH 3 ) 6 ] ⋅[Cl − ] 2 [[Co( NH 3 ) 6 ]Cl 2 ] E. [Co 2+ ] + [ NH 3 ]6 [[Co( NH 3 ) 6 ] 2+ ] 16.4. The expression of the constants of dissociation of complex ion for the compound K2[HgI4] is: А. [Hg 2+ ] ⋅ [I − ] 4 [[HgI 4 ] 2− ] [ Hg 2 + ] ⋅ 4[ I − ] [ Zn 2 + ] ⋅ [OH − ] 4 [[Zn (OH) 4 ] 2− ] 16.6. Select the most stable amminecomplex through the listed ones, if the complex ions have the given values of the constants of dissociation: А. [Cu(NH3)4]2+ Кd = 9,3·10–13 B. [Hg(NH3)4]2+ Кd = 5,2·10–20 C. [Co(NH3)6]3+ Кd = 6,2·10–36 D. [Zn(NH3)4]2+ Кd = 2,0·10–9 E. [Ni(NH3)6]2+ Кd = 1,2·10–8 16.7. What is the molecular formula of the coordination compound diammineditiocyanocopper(ІІ)? А. (NH4)2[Cu (CN)2] B. [Cu(NH3)2](CNS)2 C. [Cu(NH3)2(CNS)2] D. [Cu(NH3)2(CN)2]S E. (NH4)2[Cu (CNS)4] 16.8. What is the molecular formula of the coordination compound pentaamminebromocobalt(ІІІ) sulfate? [[ HgI 4 ] 2 − ] C. 16.5. The expression of the constants of dissociation of complex ion for the compound Na2[Zn(OH)4] is: А. [ Zn 2+ ] + [OH − ] 4 [[Zn(OH) 4 ] 2− ] [[Cu ( NH 3 ) 4 ] 2+ ] B. [K 2 [HgI 4 ]] [ K + ] 2 ⋅ [Hg 2+ ] ⋅ [ I − ] 4 [K 2 [HgI 4 ]] 78 А. [Co(NH4)5Br](SO4)2 B. [Co(NH3)5Br]SO4 C. [Co(NH3)5Br]SO3 D. [Co(NО2)5Br]SO4 E. [Co(NH3)5SO4]Br 16.9. What is the molecular formula of the coordination compound diaquadiamminenicol(ІІ) nitrateт? А. [Ni(OH)2(NH3)2](NO3)2 B. [Ni(H2O)2(NH3)2]NO2 C. [Ni(H2O)2(NH3)2]NO3 D. [Ni(H2O)2(NO3)2] E. [Ni(H2O)2(NH3)2](NO3)2 16.10. What is the molecular formula of the coordination compound potassium dihydroxotetrachlorochromate(ІІІ) А. K[Cr(OH)2Cl2] B. K3[Cr(OH)2Cl4] C. [KCrCl4](OH)2 D. K[Cr(H2O)2Cl4] E. K3[Cr(H2O)2(OH)2Cl2] 16.11. What is the molecular formula of the coordination compound dichloroaquatriamminecobalt(ІІІ) bromide? А. [Co(H2O)BrCl2(NH3)2] B. [Co(H2O)Cl2(NH3)3]Br C. [Co(NH3)3(H2O)Br]Cl2 D. [Co(H2O)Br 2(NH3)3]Cl E. [CoCl2(NH3)3H2O]Br2 16.13. What are the oxidation states of the central metal ions in the coordination compounds [Cu(NH3)4]SO4 and K2[Pt(OH)2Cl4]? А. +2, +4 B. +2, +3 C. +1, +2 D. +2, +2 E. +1, +4 16.14. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound Na3[Cr(OH)6]? А. +3, –3 B. +3, –2 C. +2, +4 D. +3, –4 E. +3, +3 16.15. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound [Co(NH3)4(CNS)2]Cl ? А. +2, 6 B. +3, 6 C. +3, 4 D. +3, 2 E. +2, 4 16.16. What are the oxidation state of the central metal ion and the charge of the complex ion for the coordination compound K2[Pt(OH)2Cl4] ? А. +4, +2 B. +4, –2 C. +4, 0 D. +2, –2 E. +2, –4 16.12. What are the oxidation states of the central metal ions in the complex ions [Fe(CN)6]4– and [Cr(H2O)4F2]+ ? А. +3, +3 B. +2, +3 C. +2, +2 D. +3, +2 E. +4, +3 79 1.1. 1.2. 1.3. 1.4. 1.5. 1.6. 1.7. 1.8. 1.9. 1.10. 1.11. 1.12. 1.13. 1.14. 1.15. 1.16. 1.17. 1.18. 1.19. 1.20. 1.21. 1.22. 1.23. 1.24. 1.25. 1.26. 1.27. 1.28. 1.29. 1.30. 1.31. 1.32. 1.33. 1.34. 1.35. 1.36. 1.37. 1.38. 1.39. 1.40. A A C E A C C E B C B E B B B E C D B E E D C D E E C E E C E E D C B D A D C B 1.41. 1.42. 1.43. 1.44. 1.45. 1.46. 1.47. 1.48. 1.49. 1.50. 1.51. 1.52. 1.53. 1.54. 1.55. 1.56. 1.57. 2.1. 2.2. 2.3. 2.4. 2.5. 2.6. 2.7. 2.8. 2.9. 2.10. 2.11. 2.12. 2.13. 2.14. 2.15. 2.16. 2.17. 2.18. 2.19. 2.20. 2.21. 2.22. 2.23. E E B C A A D A E D C D D E E B E C B C E B E E D A D C A E A A C E E E B B D A 2.24. 2.25. 2.26. 2.27. 2.28. 2.29. 2.30. 2.31. 2.32. 2.33. 2.34. 2.35. 2.36. 2.37. 2.38. 2.39. 2.40. 2.41. 2.42. 2.43. 2.44. 2.45. 2.46. 2.47. 2.48. 2.49. 2.50. 2.51. 2.52. 2.53. 2.54. 2.55. 2.56. 2.57. 2.58. 2.59. 2.60. 2.61. 2.62. 2.63. E B C D D A B E A D A E C B E E B B E A B E A D E D E E A D C C A A D E B E E B 80 2.64. 2.65. 2.66. 2.67. 2.68. 2.69. 2.70. 2.71. 2.72. 2.73. 2.74. 2.75. 2.76. 2.77. 2.78. 2.79. 2.80. 2.81. 2.82. 2.83. 2.84. 2.85. 2.86. 2.87. 2.88. 2.89. 2.90. 2.91. 2.92. 2.93. 2.94. 2.95. 2.96. 2.97. 2.98. 2.99. 2.100. 3.1. 3.2. 3.3. C C C A D D E D C B B C E A E B C E A E E D B C D B B B B D C B C E A E E C B A 3.4. 3.5. 3.6. 3.7. 3.8. 3.9. 3.10. 3.11. 3.12. 3.13. 3.14. 3.15. 3.16. 3.17. 3.18. 3.19. 3.20. 3.21. 3.22. 3.23. 3.24. 3.25. 3.26. 3.27. 3.28. 3.29. 3.30. 3.31. 3.32. 3.33. 3.34. 3.35. 3.36. 3.37. 3.38. 3.39. 3.40. 3.41. 3.42. 3.43. E E B D B D E B D B B E E B D B E B E A C C C A E C E B B E E B B B C D E E D C 3.44. 3.45. 3.46. 3.47. 3.48. 3.49. 3.50. 3.51. 3.52. 3.53. 3.54. 3.55. 3.56. 3.57. 3.58. 3.59. 3.60. 3.61. 3.62. 3.63. 3.64. 3.65. 3.66. 3.67. 3.68. 3.69. 3.70. 3.71. 3.72. 3.73. 3.74. 3.75. 3.76. 3.77. 3.78. 3.79. 3.80. 3.81. 3.82. 3.83. 3.84. 3.85. 3.86. B D A E C A A C A C C C E E A A E D A B E A C D B D C C C D D E A B E A D A E A C A B 3.87. 3.88. 3.89. 3.90. 4.1. 4.2. 4.3. 4.4. 4.5. 4.6. 4.7. 4.8. 4.9. 4.10. 4.11. 4.12. 4.13. 4.14. 4.15. 4.16. 4.17. 4.18. 4.19. 4.20. 4.21. 4.22. 4.23. 4.24. 4.25. 4.26. 4.27. 4.28. 4.29. 4.30. 4.31. 5.1. 5.2. 5.3. 5.4. 5.5. 5.6. 5.7. 5.8. B E B B B B B C C A E E D A C C D A A B B E C A B B B A C D A B B A C B C D A B E B E 5.9. 5.10. 5.11. 5.12. 5.13. 5.14. 5.15. 5.16. 5.17. 5.18. 5.19. 5.20. 6.1. 6.2. 6.3. 6.4. 6.5. 6.6. 6.7. 6.8. 6.9. 6.10. 6.11. 6.12. 6.13. 6.14. 6.15. 6.16. 6.17. 6.18. 6.19. 6.20. 6.21. 6.22. 6.23. 6.24. 6.25. 6.26. 6.27. 6.28. 6.29. 6.30. 6.31. B E A E B E C B A B C A E D A D D D B C B C A E B A B E D B C D E A A B E B A D D C D 81 6.32. 6.33. 6.34. 6.35. 6.36. 6.37. 6.38. 6.39. 6.40. 6.41. 6.42. 6.43. 6.44. 6.45. 6.46. 6.47. 6.48. 6.49. 6.50. 6.51. 6.52. 6.53. 6.54. 7.1. 7.2. 7.3. 7.4. 7.5. 7.6. 7.7. 7.8. 7.9. 7.10. 7.11. 7.12. 7.13. 7.14. 7.15. 7.16. 7.17. 7.18. 7.19. 7.20. D D B E C D C C D D B D A B A B D E E E D D C D B D B D E B C B A A E A A A D C D C A 7.21. 7.22. 7.23. 7.24. 7.25. 7.26. 7.27. 7.28. 7.29. 7.30. 7.31. 7.32. 7.33. 7.34. 7.35. 7.36. 7.37. 7.38. 7.39. 7.40. 7.41. 7.42. 7.43. 7.44. 7.45. 7.46. 7.47. 8.1. 8.2. 8.3. 8.4. 8.5. 8.6. 8.7. 8.8. 8.9. 8.10. 8.11. 8.12. 8.13. 8.14. 8.15. 8.16. A C E E A A E D E E C D B E B C B A C D C B C B E E C A A C D E C B B E D E D B D B B 8.17. 8.18. 8.19. 8.20. 9.1. 9.2. 9.3. 9.4. 9.5. 9.6. 9.7. 9.8. 9.9. 9.10. 9.11. 9.12. 9.13. 9.14. 9.15. 9.16. 9.17. 9.18. 9.19. 9.20. 9.21. 9.22. 9.23. 9.24. 9.25. 9.26. 9.27. 9.28. 9.29. 9.30. 9.31. 9.32. 10.1. 10.2. 10.3. 10.4. 10.5. 10.6. 10.7. A B B A D A C E E C D A B E B B E B A B D E D C E A B D E B D A B C E B A E D A D D D 10.8. 10.9. 10.10. 10.11. 10.12. 10.13. 10.14. 10.15. 10.16. 10.17. 10.18. 10.19. 10.20. 10.21. 10.22. 10.23. 10.24. 10.25. 10.26. 10.27. 10.28. 10.29. 10.30. 10.31. 10.32. 10.33. 10.34. 10.35. 10.36. 10.37. 10.38. 11.1. 11.2. 11.3. 11.4. 11.5. 11.6. 11.7. 11.8. 11.9. 11.10. 11.11. 11.12. B A C D A D C E D A E B D A E E D E C A C C B B E A C B B E E B D C C D E C C C B C D 11.13. 11.14. 11.15. 11.16. 11.17. 11.18. 11.19. 11.20. 11.21. 12.1. 12.2. 12.3. 12.4. 12.5. 12.6. 12.7. 12.8. 12.9. 12.10. 12.11. 12.12. 12.13. 12.14. 12.15. 12.16. 12.17. 12.18. 12.19. 12.20. 12.21. 12.22. 12.23. 12.24. 12.25. 12.26. 12.27. 12.28. 12.29. 12.30. 12.31. 12.32. 12.33. 12.34. C E A C B D B C C A E A B E B A B E A D B D E B B B E C C B C B A D C A A A B E A D C 82 12.35. 12.36. 12.37. 12.38. 12.39. 12.40. 12.41. 12.42. 12.43. 12.44. 13.1. 13.2. 13.3. 13.4. 13.5. 13.6. 13.7. 13.8. 13.9. 13.10. 13.11. 13.12. 13.13. 13.14. 13.15. 13.16. 13.17. 13.18. 13.19. 13.20. 13.21. 13.22. 13.23. 13.24. 13.25. 13.26. 13.27. 13.28. 13.29. 13.30. 13.31. 13.32. 13.33. E E B B A D D A B C C E B E B E E C D D E E A C A B A D D C A E A E A E B C E D E B D 13.34. 13.35. 13.36. 13.37. 13.38. 13.39. 13.40. 13.41. 13.42. 13.43. 13.44. 13.45. 13.46. 13.47. 13.48. 13.49. 13.50. 13.51. 13.52. 13.53. 13.54. 13.55. 13.56. 14.1. 14.2. 14.3. 14.4. 14.5. 14.6. 14.7. 14.8. 14.9. 14.10. 14.11. 14.12. 14.13. 14.14. 14.15. 14.16. 15.1. 15.2. 15.3. 15.4. E C E A E A A E C C A B B C B E B D D D C A E E D D A D E C B C A D D A A D C A D B C 15.5. 15.6. 15.7. 15.8. 15.9. 15.10. 15.11. 15.12. 15.13. 15.14. 15.15. 15.16. B B C B C C C A E A B C 15.17. 15.18. 15.19. 15.20. 15.21. 15.22. 15.23. 15.24. 15.25. 15.26. 15.27. 15.28. C A A A E A E A D D D A 15.29. 15.30. 15.31. 15.32. 15.33. 15.34. 15.35. 15.36. 15.37. 15.38. 15.39. 15.40. E A D D A B D C D D D C 83 15.41. 15.42. 15.43. 15.44. 15.45. 15.46. 16.1. 16.2. 16.3. 16.4. 16.5. 16.6. D E B D E C A A C A E C 16.7. 16.8. 16.9. 16.10. 16.11. 16.12. 16.13. 16.14. 16.15. 16.16. C B E B B B A A B B CONTENTS Chapter 1. Atomic-molecule concept. Nomenclature and classification of inorganic compounds ................................................................................. 2 Chapter 2. Structure of atoms. The Periodic law and Periodic table by D. Mendeleev ................................................................................................. 7 Chapter 3. Equivavlents of substances in chemical reactions .................................... 17 Chapter 4. Solutions. Ways of expressing concentration of solutions ....................... 27 Chapter 5. Colligative properties of solutions ........................................................... 31 Chapter 6. The basic terms of chemical thermodynamics. Thermochemistry. The direction of chemical processes passage ........................................... 33 Chapter 7. The rate and mechanism of chemical reactions........................................ 39 Chapter 8. The equilibrium in feebly soluble electrolytes solutions .......................... 45 Chapter 9. Chemical equilibrium............................................................................... 48 Chapter 10. The concept of strong and weak electrolytes ......................................... 53 Chapter 11. Acids and bases theories. Self-ionization of water. pH .......................... 56 Chapter 12. Protolytic processes................................................................................ 59 Chapter 13. Reactions with electrons transferring ..................................................... 63 Chapter 14. Experimental studying of reduction-oxidation reactions ....................... 70 Chapter 15. Coordination compounds. Reactions of coordination compounds formation ................................................................................................. 72 Chapter 16. Experimental studying of coordination compounds properties .............. 77 84