Lab - Molar Mass of a Volatile Liquid
advertisement

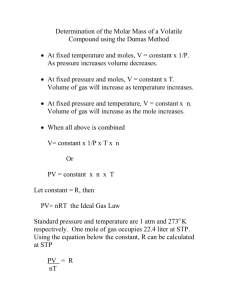
Molar Mass of a Volatile Liquid It is often useful to know the molecular mass of a substance. This is one of the properties that helps characterize the substance. If the substance is a volatile liquid, one common way of determining its molecular mass involves using the ideal gas law, PV = nRT. Since the liquid is volatile, it can easily be converted to a gas. While it is in the gas phase, its volume, temperature and pressure are measured. The ideal gas law will then allow the calculation of the number of moles of the substance present: n= PV RT In this equation, n is the number of moles of gas, P is pressure, V is volume, R is the ideal gas constant (0.0821 L⋅atm/mole⋅K), and T is the temperature on the Kelvin scale. The molar mass in grams, M, is equal to the mass g of the gas sample divided by the number of moles n. M = g/n In this experiment to determine the molecular mass of a volatile liquid, a small amount of the liquid is introduced into a weighed flask. The flask is then placed in boiling water, where the liquid will vaporize completely, driving out the air and filling the flask with vapor at barometric pressure and the temperature of the boiling water. If we cool the flask so that the vapor condenses, we can measure the mass of the vapor and calculate a value for M. Chemical & Equipment unknown volatile liquid containing I2 125-mL Erlenmeyer flask aluminum foil rubber band pin thermometer beaker, 600-mL hot plate analytical balance Procedure 1. Prepare the Flask for the Sample. Obtain a clean, dry 125-mL Erlenmeyer flask. Determine the mass of the “empty” flask to the nearest 0.001 g. Cover the flask with a small piece of aluminum foil and secure it with a rubber band; do not use any excess foil as it will trap water. Determine the mass of the dry flask, aluminum foil, and rubber band to the nearest 0.001 g. 2. Place the Sample in the Flask. Transfer about 5 mL of the unknown liquid into the flask; again cover the flask with the aluminum foil and secure the foil with a rubber band. You do not need to conduct a mass measurement. With a pin, pierce the aluminum foil several (6-10) times. 3. Prepare a Boiling Water Bath. Fill a 600-mL beaker ~2/3 with water, place on hot plate, and bring to a gentle boil. 4. Place the Flask/Sample in the Bath. Lower the flask/sample into the bath and use a utility clamp to hold the flask under water up to the foil. Don’t clamp around the neck of the flask—use the clamp as a weight on top. Try to keep the flask from touching the beaker wall (so it doesn’t overheat). 5. Heat the Sample to the Temperature of Boiling Water. Heat the water until it reaches a gentle boil. When the liquid in the flask or the vapors escaping from the holes in the aluminum foil are no longer visible, continue heating for another 5 minutes. Read and record the temperature of the boiling water. [However, rather than use your thermometer reading, we will assume as long as the water is boiling, it’s temperature is 100.0°C.] 6. Measure the Mass of the Flask/Sample. Remove the flask and allow it to cool to room temperature. Sometimes the remaining vapor in the flask condenses; that's O.K. Dry the outside of the flask and determine the mass of the flask, aluminum foil, rubber band, and the vapor. 7. Do It Again. Repeat the experiment for Trial 2. You only need to transfer another 5 mL of liquid to the flask. 8. Measure the Volume of the Flask. Fill the empty 125-mL Erlenmeyer flask to the brim with water. Weigh the contents in a Styrofoam cup; you may have to do it in two batches. 9. Record the Pressure of the Vapor in the Flask. Find the barometer in the laboratory. Disposal: Dispose of the leftover unknown liquid in the "Waste Organics" container in the hood. Calculations Calculate the molar mass of the liquid. Find the identity of the unknown liquid from the teacher. Your result should be within 10% of the actual molar mass. If your percent error is greater than 10%, propose explanations.