Worksheet 4.2 answers
advertisement
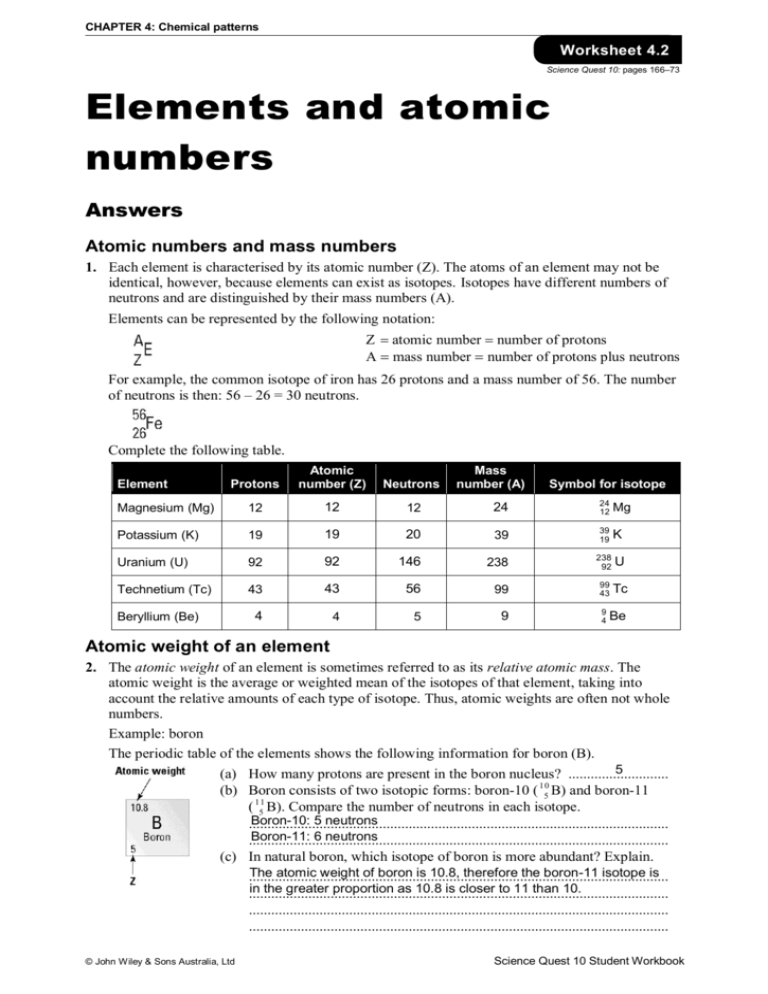
CHAPTER 4: Chemical patterns Worksheet 4.2 Science Quest 10: pages 166–73 Elements and atomic numbers Answers Atomic numbers and mass numbers 1. Each element is characterised by its atomic number (Z). The atoms of an element may not be identical, however, because elements can exist as isotopes. Isotopes have different numbers of neutrons and are distinguished by their mass numbers (A). Elements can be represented by the following notation: Z atomic number number of protons A mass number number of protons plus neutrons For example, the common isotope of iron has 26 protons and a mass number of 56. The number of neutrons is then: 56 – 26 = 30 neutrons. Complete the following table. Protons Atomic number (Z) Neutrons Magnesium (Mg) 12 12 12 24 24 12 Mg Potassium (K) 19 19 20 39 39 19 K Uranium (U) 92 92 146 238 238 92 U Technetium (Tc) 43 43 56 99 99 43 Tc 4 4 5 9 Element Beryllium (Be) Mass number (A) Symbol for isotope 9 4 Be Atomic weight of an element 2. The atomic weight of an element is sometimes referred to as its relative atomic mass. The atomic weight is the average or weighted mean of the isotopes of that element, taking into account the relative amounts of each type of isotope. Thus, atomic weights are often not whole numbers. Example: boron The periodic table of the elements shows the following information for boron (B). 5 (a) How many protons are present in the boron nucleus? ........................... 10 (b) Boron consists of two isotopic forms: boron-10 ( 5 B) and boron-11 ( 115 B). Compare the number of neutrons in each isotope. Boron-10: 5 neutrons ................................................................................................................. Boron-11: 6 neutrons ................................................................................................................. (c) In natural boron, which isotope of boron is more abundant? Explain. The atomic weight of boron is 10.8, therefore the boron-11 isotope is ................................................................................................................. in the greater proportion as 10.8 is closer to 11 than 10. ................................................................................................................. ................................................................................................................. ................................................................................................................. © John Wiley & Sons Australia, Ltd Science Quest 10 Student Workbook




