Potential Energy Diagrams Worksheet: Chemistry Practice
advertisement
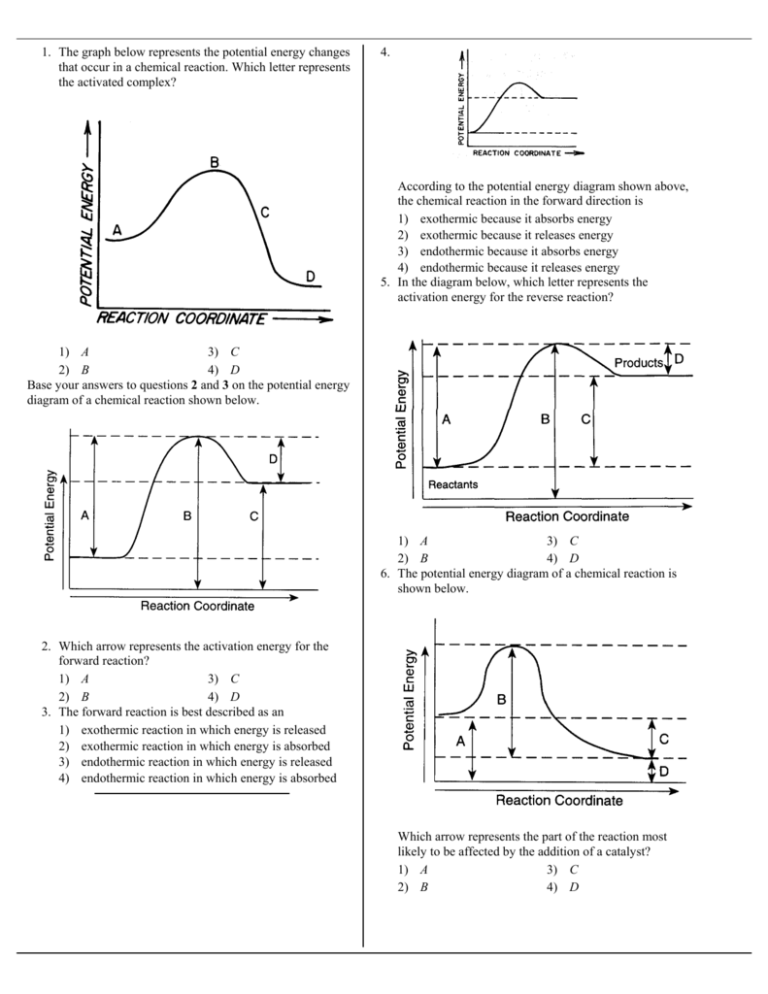
1. The graph below represents the potential energy changes that occur in a chemical reaction. Which letter represents the activated complex? 4. According to the potential energy diagram shown above, the chemical reaction in the forward direction is 1) exothermic because it absorbs energy 2) exothermic because it releases energy 3) endothermic because it absorbs energy 4) endothermic because it releases energy 5. In the diagram below, which letter represents the activation energy for the reverse reaction? 1) A 3) C 2) B 4) D Base your answers to questions 2 and 3 on the potential energy diagram of a chemical reaction shown below. 1) A 3) C 2) B 4) D 6. The potential energy diagram of a chemical reaction is shown below. 2. Which arrow represents the activation energy for the forward reaction? 1) A 3) C 2) B 4) D 3. The forward reaction is best described as an 1) exothermic reaction in which energy is released 2) exothermic reaction in which energy is absorbed 3) endothermic reaction in which energy is released 4) endothermic reaction in which energy is absorbed Which arrow represents the part of the reaction most likely to be affected by the addition of a catalyst? 1) A 3) C 2) B 4) D 7. The diagram below represents the energy changes that occur during the formation of a certain compound under standard conditions. 9. Which graph represents an endothermic reaction? 1) 3) 2) According to Reference Table I, the compound could be 1) C2H6(g) 2) CO2(g) 3) HI(g) 4) NH3(g) 8. The potential energy diagram below represents a reaction. 4) 10. Which diagram represents the potential energy of an exothermic reaction? 1) 3) 2) 4) 11. The potential energy diagram below shows the reaction X + Y → Z. Which arrow represents the activation energy of the forward reaction? 1) A 3) C 2) B 4) D When a catalyst is added to the reaction, it will change the value of 1) 1 and 2 3) 2 and 3 2) 1 and 3 4) 3 and 4 12. Given the reaction: S(s) + O2(g) - SO2(g) + energy 13. The potential energy diagram below represents the reaction 2KClO3 → 2KCl + 3O2. Which diagram best represents the potential energy changes for this reaction? 1) 2) 3) Which numbered interval on the diagram would change when a catalyst is added? 1) 1 3) 3 2) 2 4) 4 14. Which information about a chemical reaction is provided by a potential energy diagram? 1) the oxidation states of the reactants and products 2) the average kinetic energy of the reactants and products 3) the change in solubility of the reacting substances 4) the energy released or absorbed during the reaction 15. In the potential energy diagram below, which letter represents the potential energy of the activated complex? 4) 1) A 3) C 2) B 4) D 16. In a potential energy diagram, the difference between the potential energy of the products and the potential energy of the reaction is equal to the 1) heat of reaction 2) entropy of the reaction 3) activation energy of the foward reaction 4) activation energy of the reverse reaction 17. The graph below represents a chemical reaction. This reaction is best described as 1) endothermic, because energy is absorbed 2) endothermic, because energy is released 3) exothermic, because energy is absorbed 4) exothermic, because energy is released 18. A potential energy diagram is shown below. Which letters represent the activation energy of the forward and reverse reactions, respectively? 1) A and C 2) A and D 3) B and C 19. The activation energy required for a chemical reaction can be decreased by 1) increasing the surface area of the reactant 2) increasing the temperature of the reactant 3) adding a catalyst to the reaction 4) adding more reactant 4) B and D 20. Which interval on the potential energy diagram shown below represents the ∆H of the reaction a + b → c + d? 1) 1 3) 3 2) 2 4) 4 21. According to the potential energy diagram below, what is the reaction A + B → C? Base your answers to questions 22 and 23 on the reaction coordinate shown below: 22. Which interval represents the heat of reaction? 1) A 3) C 2) B 4) D 23. Which interval represents the activation energy of the forward reaction? 1) A 3) C 2) B 4) D 24. The potential energy diagram of a chemical reaction is shown below. 1) 2) 3) 4) endothermic and ∆H is positive endothermic and ∆H is negative exothermic and ∆H is positive exothermic and ∆H is negative Which letter in the diagram represents the heat of reaction (∆H)? 1) A 3) C 2) B 4) D Base your answers to questions 25 and 26 on the potential energy diagram below, which represents the reaction: A + B → C + energy. 25. Which numbered interval will change with the addition of a catalyst to the system? 1) 1 3) 3 2) 2 4) 4 26. Which statement correctly describes this reaction? 1) It is endothermic and energy is absorbed. 2) It is endothermic and energy is released. 3) It is exothermic and energy is absorbed. 4) It is exothermic and energy is released. 28. A potential energy diagram is shown below. Which reaction would have the lowest activation energy? 1) the forward catalyzed reaction 2) the forward uncatalyzed reaction 3) the reverse catalyzed reaction 4) the reverse uncatalyzed reaction 29. Given the potential energy diagram: 27. Given the reaction: N2(g) + 2O2(g) ↔ 2NO2(g) ∆H = + 7.9 kcal/mole The potential energy diagram of the reaction is shown below. With reference to energy, the reaction A + B → AB can best be described as 1) endothermic, having a +∆H 2) endothermic, having a –∆H 3) exothermic, having a +∆H 4) exothermic, having a –∆H Which arrow represents the heat of reaction (∆H) for the reverse reaction? 1) 1 3) 3 2) 2 4) 4 30. Given the potential energy diagram of a chemical reaction: Which arrow represents the potencial energy of the reactants? 1) A 3) C 2) B 4) D 31. The graph below is a potential energy diagram of a compound which is formed from its elements. 32. A potential energy diagram of a chemical reaction is shown below. What is the difference between the potential energy of the reactants and the potential energy of the products? 1) 20. kcal 3) 60. kcal 2) 40. kcal 4) 80. kcal 33. According to Table I, which potential energy diagram best represents the reaction that forms H2O(…) from its elements? 1) 3) 2) Which interval represents the heat of reaction? 1) A 3) C 2) B 4) D 4) 34. When a spark is applied to a mixture of hydrogen and oxygen, the gases react explosively. Which potential energy diagram best represents the reaction? 1) 35. Base your answers on the potential energy diagram below. 2) 3) The potential energy of the activated complex is equal to the sum of 1) X + Y 3) X + Y + W 2) X + W 4) X + W + Z 36. In the potential energy diagram below, which interval represents the heat of reaction (∆H)? 4) 1) A 2) B 3) C 4) D Reference Tables Answer Key 1. 2 30. 2 2. 1 31. 4 3. 4 32. 1 4. 3 33. 1 5. 4 34. 2 6. 2 35. 2 7. 3 36. 3 8. 2 9. 3 10. 4 11. 3 12. 1 13. 2 14. 4 15. 2 16. 1 17. 4 18. 3 19. 3 20. 2 21. 1 22. 2 23. 3 24. 2 25. 2 26. 4 27. 2 28. 1 29. 4








