How to Read the Periodic Table: A Beginner's Guide
advertisement
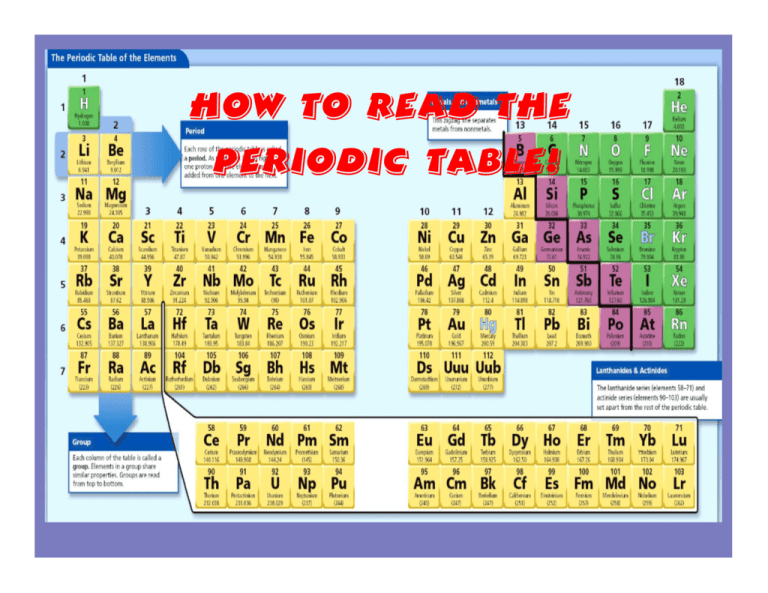
How to Read the Periodic Table! How to Identify things on a periodic table Atomic Number indicates the # of _______ Chemical Symbol Element Name Atomic Mass indicates the # of _______ & _______ How to Read the Periodic Table: -Broken into 18 groups & 7 periods. *REMEMBER: groups- vertical periods- horizontal -Groups are related by chemical and physical properties, whereas periods are not. *Example: Group 17 (Halogens) In group 17, the elements show many similarities. Members of the group can be similar, but not identical. How to Read the Periodic Table by Group/ Period (Open Textbooks to p. 20): -So if I were to say find the element at group 11, period 5... Find your key! This will help you determine the element's details. :) NOTE: not every periodic table is the same! Make sure you identify the key. How to Read the Periodic Table by Group/ Period (Open Textbooks to p. 20): Let's Try a Few Together! :) 1. Find the element at group 14, period 2. CARBON 2. Find the element at group 17, period 5. IODINE 3. In what group/period would we find Lithium? Group 1, Period 2. 4. In what group/period would we find Neptunium? Group 3, Period 7 5. Find the element at group 8, period 4. IRON How to Read the Periodic Table by Group/ Period (Open Textbooks to p. 20): Let's Try a Few Together! :) 1. Find the element at group 14, period 2. What is the atomic number of this element? Carbon- 6 2. Find the element at group 17, period 5. What is the chemical symbol of this element? Iodine- I 3. Find Lithium on the periodic table. What state of matter is it at room temperature? Solid 4. Find the element in Group 3 Period 7. Is it a metal, non metal, or metalloid? Metal 5. Find the element at group 8, period 4. What is its atomic mass? Iron- 55.845



