S. Hessam Moosavi Mehr: Organic Chemistry in a Nutshell
advertisement

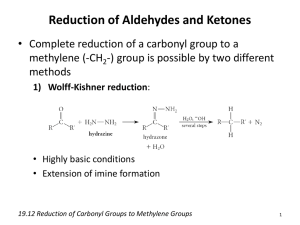
Organic Chemistry in a Nutshell S. Hessam Moosavi Mehr Spring 2008 Remember, this is not a textbook! If you do not understand a term, refer to the glossary at the end of the document. Contents Review Structure Transformation Using Curved Arrows Rule I: Base-Acid Interaction . . . . . . Rule II: Bond Cleavage . . . . . . . . . . Rule III: Bond Transfer . . . . . . . . . . Flying chunks . . . . . . . . . . . . . . . Resonance and Resonance Effects . . . . . . . . Finding Reasonable Reaction Mechanisms . . . Reaction Energetics . . . . . . . . . . . . . . . . 1 1 1 2 2 2 2 2 3 Stereochemistry Showing Molecules in 3D . . . Perspective Formula . Newman Projection . Sawhorse Projection . Fischer Structure . . . Configurations . . . . . . . . . Rule I . . . . . . . . . . Rule II . . . . . . . . . Multiple Bonds . . . . Configuration in Alkenes . . . Stereochemistry in Reactions . . . . . . . . . . . . 3 3 3 3 3 3 4 4 4 4 4 4 Radical Initiated Reactions NBS . . . . . . . . . . . . . . . . . . . . . . . . . 4 4 Substitution and Elimination Reactions Leaving groups . . . . . . . . . . . . . . . . . . . Alcohol Dehydration . . . . . . . . . . . . . . . Hofmann Elimination . . . . . . . . . . . . . . . 4 4 5 5 Review . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Reactions of the Carbon-Carbon Double Bond Halogen/Hydrohalogen Addition . . . . . Classic/Free Carbocations . . . . . Kinetic / ermodynamic Control Addition of Acids . . . . . . . . . . . . . . Peroxide Impurities . . . . . . . . . Hydrogenation . . . . . . . . . . . . . . . . Hydration and Addition of Alcohols . . . . Oxymercuration/Demercuration . Anti-Markovnikov Hydration . . . Epoxidation . . . . . . . . . . . . . . . . . Reactions of Epoxides . . . . . . . Diol Production . . . . . . . . . . . . . . . Co-oxidants . . . . . . . . . . . . . Oxidative Cleavage . . . . . . . . . . . . . Permanganate Cleavage . . . . . . Ozonolysis . . . . . . . . . . . . . . Addition of Carbenes . . . . . . . . . . . . StructureTransformationUsingCurvedArrows . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5 5 5 5 5 5 5 5 5 5 6 6 6 6 6 6 6 6 Reactions of Alcohols and Carbonyl Compounds Oxidation . . . . . . . . . . . . . . . . . . . . Aldol Condensation . . . . . . . . . . . . . . . e Wittig Reaction . . . . . . . . . . . . . . . e Grignard Reaction . . . . . . . . . . . . . Reductions . . . . . . . . . . . . . . . . . . . . Wolff-Kischner Reduction . . . . . . . Clemmensen Reduction . . . . . . . . Reduction using Hydride . . . . . . . . . . . . . . . . 6 6 7 7 7 7 7 7 7 Organic Synthesis Describing a Synthesis . . . . . . . . . . . . . . Retrosynthesis . . . . . . . . . . . . . . . . . . . 7 7 8 Glossary 8 References for Further Reading 8 . . . . . . . . . . . . . . . . . RuleI:Base-AcidInteraction You usually begin using an arrow of this type. is type of arrow always originates from a lone pair. Curved arrows allow us to illustrate the mechanism for a reaction. A curved arrow shows the direction of flow for a pair of electrons. It always starts from the electron source (Lewis base) and terminates at the sink (Lewis acid). All you need to know in order to be able to draw curved arrows is the following three rules. A B A B Here is an example: the addition of water to a carbocation 1 H H H O H H O H H O H H H O RuleII:BondCleavage ResonanceandResonanceEffects is type of arrow signifies the heterogeneous breaking of a bond. It usually occurs as the last arrow in a succession. e direction of the arrow is very important. As always, it shows the flow of electrons. Resonance can stabilize a species with respect to polar reactions by spreading localized charges over the molecule. is can effectively prevent a base from protonation or an acid from giving up protons. A B A B Here is an example illustrating type I and type II interactions. O H O H O O H O H O Methoxide anion To see how effective this can be, compare methanol (pKa = 15.9) to acetic acid (pKa = 4.8). is huge difference in acidity results from the relative stabilites of their conjugate bases. e acetate ion can spread its negative charge over two oxygen atoms while in the methoxide anion, the negative charge is bound to the single oxygen atom. H RuleIII:BondTransfer is is the most common type of arrow. It signifies the transfer of a bonding pair. e number of electrons around C increases by 2 and the number of electrons around decreases by 2. e number of electrons around A remains unchanged. FindingReasonableReactionMechanisms • A reaction mechanism usually consists of several steps. • A step is a series of transformations that happen at the same time. A B C • For each step, curved arrows show the movement of electron [pairs]. A B Acetate anion H C • Separate steps using or → depending on whether the transformation is reversible or irreversible. Flyingchunks In rearrangement reactions, some steps may involve groups that are “flying” from one moiety to the other. Here is an example from the cummene rearrangement. Flying moieties also play key roles in carbocation rearrangements, the Hofmann degradation reaction, pinacol rearrangement, Lossen rearrangement, Wolff rearrangement, and Baeyer-Villiger oxidation. • Steps that require the collision of three or more molecules are not feasible. Consider revising you reaction mechanism if it comprises such collisions. • Each step should produce a compound which does not violate the octet rule for elements from periods 1 and 2. 2 ReactionEnergetics A typical reaction has transition states and intermediates associated with it. NewmanProjection e Newman projection is most useful when studying the relative orientation of the various groups attached to two adjacent carbon atoms and how their interaction varies depending on their dihedral angles. H H H H H H Newman projection showing a staggered conformer for ethane Hammond’s principle states that the transition state is structurally closer to the species which more closely mimics it energetically. is means that in highly exothermic reactions, the transition state resembles the reactant most closely, while in highly endothermic reactions, the transition state closely mimics the product, as shown below. SawhorseProjection is is similar to the perspective formula except that it uses long inclined solid lines to replace wedges. H H3C H H H3C H Sawhorse projection showing an eclipsed conformer for butane Transition state ≈ product FischerStructure Stereochemistry Each carbon atom is held in a way so that the two groups sticking out of the plane lie horizontally while the ones which lie behind the plane lie vertically. ShowingMoleculesin3D ere are generally 4 methods for displaying the stereochemistry of a molecule. COOH PerspectiveFormula OH At any given orientation, the central carbon atom and two of its bonds lie entirely within a plane. Of the other two bonds one sticks out of the plane toward you —this you show using a solid wedge— and one goes behind the plane, which is designated by a hatched wedge. is is the most common way of showing species in 3D. HO COOH (R,R)-tartaric acid 3 RadicalInitiatedReactions Configurations To describe the configuration around a carbon atom in the Cahn-Ingold-Prelog system, hold the chiral carbon atom in such a way that the group with the lowest priority faces away from you. en trace a path that connects the other three groups in order of decreasing priority. If the path goes clockwise, the configuration is R, otherwise the carbon is said to have S configuration. Priorities are assigned according to the following rules. F Cl Br 1◦ 1 1 1 2◦ ∼1 3.8 82 3◦ ∼1 5 1600 NBS O RuleI N Br Of two atoms, the one with the higher atomic number has a higher priority. Of two atoms with the same atomic number, the one with the higher atomic mass has a higher priority. O N-bromosuccinimide is often used as a convenient laboratory substitute for bromine in cases where the hydrocarbon is allylic, benzylic, or the bromination of the α carbon in a carbonyl compound is desired. RuleII If rule I results in a tie¹, study the atoms attached to each. Cancel similar atoms and repeat as necessary. NBS Br Br2 MultipleBonds Radical bromination of propene at allylic position Replace multiple bonds with the equivalent structure in the picture below. SubstitutionandEliminationReactions Leavinggroups Generally speaking, a moiety is considered a better leaving group if it is a weaker base. For example, acetate is a vastly better leaving group than hydroxyl, because the acetate ion is a much weaker base than hydroxide. e presence of a bad leaving group makes both substitution and elimination very difficult. Fortunately, there are methods available for converting a bad leaving group to a good one. One widely used solution for alcohols is treatment with tosyl chloride. ConfigurationinAlkenes e E/Z system of nomenclature can be used to differentiate between the geometrical isomers of an alkene. According to this system, the group with the higher priority attached to each carbon of the double bond is identified. e alkene is then designated with and “Z-” if the groups of higher priority lie on the same side, and “Z-” otherwise as can be seen in the example below. O S Cl O HO (E)-Stilbene StereochemistryinReactions O An addition or elimination reaction is called syn if the groups to be added/eliminated do so from the same side of the molecule as in the addition of hydrogen. In an anti reaction, however, the addition/elimination occurs from opposite faces of the molecule as in the addition of bromine to alkenes in peroxide free environments. O S O Conversion of ethanol to ethyl tosylate is converts the bad hydroxide leaving group to the very good tosylate (TsO− ). ¹at is, if it cannot decide which of the two groups has higher priority 4 AlcoholDehydration AdditionofAcids e mechanism is E2 for primary alcohols, E1 for secondary and tertiary alcohols. e most stable alkene, the most substituted one, is formed. is reaction is thus said to follow Zaitsev’s rule. Dehydration for secondary alcohols is commonly accompanied by rearrangements involving 1,2-hydride or 1,2-alkyl shifts in order to form more stable 3◦ carbocation intermediates. Ether formation can become substantial in some cases if conditions are not controlled carefully. e addition of halo-acids proceeds according to Markovnikov’s rule and is not stereospecific. PeroxideImpurities If peroxide impurities exist in the reaction environment, the addition of halo-acids will shift to a radical mechanism which produces the anti-Markovnikov product. HofmannElimination Hydrogenation e least substituted alkene, although not the most stable possible product, is the major product in this reaction. is is contrary to Zaitsev’s rule and is called the Hofmann rule. N Syn hydrogenation of a double bond can be effected through the use of a catalyst, most commonly platinum, palladium, or nickel. Also of interest is the so-called Wilkinson’s Catalyst, RhCl(P3 )3 . HydrationandAdditionofAlcohols AgO Treating an alkane with acid in aqueous environment will yield an alcohol. e reaction is not stereospecific because it involves a planar carbocation. It is regioselective however and follows Markovnikov’s rule, which means that the most stable carbocation intermediate is produced in the first step. If the procedure is carried out in alcohol as a solvent, an ether is formed as expected. ReactionsoftheCarbon-CarbonDoubleBond Halogen/HydrohalogenAddition Halogens, most commonly bromine, bring about anti addition when reacting with alkenes. e reaction normally proceeds through a triangular cationic species that guarantees the anti stereochemistry of the addition. Oxymercuration/Demercuration e problem with the above procedure is that it inevitably involves a carbocation. is brings with it complications like rearrangement and polymerization. On a laboratory scale where product purity is of utmost importance, there is an alternative synthesis, oxymercuration/demercuration, which avoids carbocations altogether, thereby preventing rearrangements, etc. In this procedure, the alkene is treated with mercury (II) acetate, then reduced with sodium borohydride. is latter step involves radicals and as a result, the reaction is not stereospecific. Br Br Br Br Br Br BrClassic/FreeCarbocations e formation of the triangular intermediate in halogen addition prevents the formation of a carbocation. is however introduces severe strain as it involves a threemembered ring². In cases where the would-be carbocation is stable enough, the cyclic does not form, inevitably resulting in loss of stereoselectivity. NBS in aqueous environment can also be used to the same effect. Anti-MarkovnikovHydration Anti-Markovnikov hydration can be achieved through borohydration-oxidation. is reaction is yet another example of alkyl group migration. Borohydrationoxidations is the only common hydration reaction which is stereospecific. It follows a syn mechanism, i.e. the hydrogen and hydroxide add to opposite sides of the molecular plane. Kinetic/ThermodynamicControl e addition of bromine to butadiene will yield two products. Exactly how much of each is produced depends on the temperature at which the reaction is carried out. ²is is often called a classical carbocation. 5 Epoxidation PermanganateCleavage Reacting an alkene with a dilute acidic solution of permanganate will result in the cleavage of the alkene at the double bond. CRR′ groups will produce ketones; CHR groups yield carboxylic acids; and CH2 groups give carbon dioxide. e fact the reagent over-oxidizes 1◦ groups and methylenes is a major downside with this reaction. Treating an alkene with an organic peracid, such as mCPBA (meta-chloroperbenzoic acid) or benzoyl peroxide through the so-called butterfly mechanism. It happens in a single step, i.e it is a concerted reaction. Cl O O O O Ozonolysis H e reaction of an alkene with ozone, followed by hydrolysis will break the alkene in two. e advantage of such a treatment is that oxidation does not proceed further than aldehyde/ketone production. Ozone is expensive and difficult to handle, though, and it is best to avoid the reaction in cases where permanganate produces satisfactory results. ReactionsofEpoxides Epoxide rings can be opened under both acidic and basic conditions. Under acidic conditions, there is substantial development of positive charge, which favors nucleophilic attack on the carbon atom which would produce the most stable carbocation. is is called a pseudo-SN1 mechanism. In a basic solution, however, there is little development of positive charge, and the nucleophile attacks the least crowded carbon atom. is is called a pseudo-SN2 ) mechanism. AdditionofCarbenes Carbenes, CRR′ are unstable organic species. eir usefulness in organic chemistry is primarily due to the fact that they can add to a carbon-carbon double bond to produce a three-membered ring. Carbenes can exist in singlet and triplet forms. It is found that the singlet form (s = 1) always produces syn addition while the triplet form (s = 3) does not react in a stereospecific way and is thus undesirable. Molecular orbital arguments can show that the species favors the singlet form if R and R′ contain lone pairs. is is why we often use dicholorocarbene„ CCl2 , instead of carbene itself (CH2 ). e addition of base to trichloroacetic acid or chloroform can provide dichlorocarbene on a laboratory scale. Another possibility is the addition of zinc to diiodomethane, CH2 I2 , which seems to produce singlet carbene exclusively. is is called the Simmons-Smith reaction. DiolProduction An alkene can be converted to a vicinal diol when treated with either of the following • Osmium tetroxide (OsO4 ) • Cold, dilute, potassium permanganate in neutral environment Co-oxidants Osmium tetroxide, while offering excellent performance in the production of diols from olefins, is toxic and extremely expensive. It is thus best used as catalyzer, converting the alkene to a diol and itself being reduced in the process. A reagent called the co-oxidant then oxidizes osmium back to its (VIII) state, whereupon it continues the diol production process. A common choice for co-oxidant is N-methylmorpholine-N-oxide, or NMO for short, shown below. ReactionsofAlcoholsandCarbonyl Compounds Oxidation e most common reagent for oxidizing alcohols is chromium (VI) in acid, often called the Jones reagent. Treatment with dilute permanganate in neutral pH can also effect oxidation in cases when acids are to avoided. Tertiary alcohols cannot be oxidized “peacefully” under ordinary conditions. Oxidation of secondary alcohols yields ketones. Primary alcohols can be converted to aldehydes when treated with PCC or PDC in a nonaqueous medium. O O N NMO OxidativeCleavage ere are a host of methods available for oxidative cleavage of both bonds in the double junction of an olefin. Here we will examine ozonolysis and permanganate cleavage. 6 ketones will give 3◦ alcohols. Because 2◦ and 3◦ alcohols can easily be dehydrated to olefins, the Grignard reaction also opens the path to a plethora of exotic reactions possible with alkenes. Here is how benzyl alcohol can be prepared from formaldehyde through the Grignard reaction. Pyridinium chlorochromate e presence of water should be avoided if an aldehyde is desired, as water converts the aldehyde to a geminal diol, which will further be oxidized to a carboxylic acid. Oxidation of primary alcohols in water yields carboxylic acids, except for methanol which turns to carbon dioxide. O H + H AldolCondensation Aldol condensation is useful because it allows us to create carbon-carbon bonds without the use of “exotic” chemicals. Here is an example OH- O OH PhMgBr Afterwards: H2O + H+ Reductions e carbonyl group in an aldehyde/ketone can be reduced to a hydroxyl group or converted to a methylene (CH2 ) depending on the reagent used. O Soxhelt Wolff-KischnerReduction TheWittigReaction Treating a carbonyl compound with hydrazine (N2 H4 in strongly basic environment converts the carbonyl to methylene. is method works best with aromatic or vinylic compounds. Another valuable reaction is the Wittig reaction, named after the German Georg Wittig, which allows the conversion of a carbonyl group to a carbon-carbon double bond. is reaction employs a phosphorus containing species known as an ylide. Many variations have since been devised, such as the Horner reaction. e mechanism for this reaction has also been the subject of considerable debate. e classical mechanism involves two intermediate species, known as the betaine and the oxaphosphetane. e little-known Lombardo reaction may sometimes be used as an alternative to the Wittig reaction. ClemmensenReduction Treating a carbonyl compound with zinc amalgam and acid reduces aldehydes and ketones completely to methylene while leaving carboxyl groups intact. ReductionusingHydride Sodium borohydride (NaBH4 ) and lithium aluminum hydride (LiAlH4 ) can both reduce aldehydes and ketones to alcohols. Lithium aluminum hydride is so reactive that can also reduce esters and carboxylic acids. Sodium borohydride is the prefered reagent for aldehydes and ketones because it is safer and easier to handle and does not promote side-reactions. O Ph P Ph Ph OrganicSynthesis Ph P Ph O Ph P Ph Ph DescribingaSynthesis O When asked to devise a synthesis for a compound, you are either given a molecule to begin with or not. In the first case, write the given molecule then connect it in a succession ultimately leading to the desired end-product. Above and below each arrow, describe which reagents and conditions the transformation will demand. You shouldn’t draw curved arrows or describe mechanisms unless you are explicitly asked to. Ph Ph + O P Ph Ph An example Wittig reaction TheGrignardReaction Starting material Like aldol condensation, the Grignard reaction creates carbon-carbon bonds. It converts a carbonyl compound to an alcohol. If formaldehyde is used, a 1◦ alcohol results. Any other aldehyde will produce a 2◦ alcohol, while X 7 Y 1: a,b 2: c,d Heat, etc. e, f in N2 Final product Z Retrosynthesis • Robert T. Morrison, Robert N. Boyd, Organic Chemistry, Prentice Hall, 6th Edition, 1992 A common way of devising a synthesis for a compound is to successively remove the the parts that are straightforward to attach using the reactions available. Interested readers may refer to Warren’s book cited in the references. • Paula Y. Bruice, Organic Chemistry, Prentice Hal, 5th Edition, 2006 • Seyhan Eğe, Organic Chemistry: Structure and Reactivity, Houghton Mifflin Company, 5th Edition, 2003 Glossary Aldol Aldehyde + Alcohol • Francis A Carey, Organic Chemistry, McGrawHill Science/Engineering/Math, 7th Edition, 2007 Condensation A condensation reaction is an addition, followed by the elimination of a small, trivial, molecule like water or an alcohol. Examples are aldol condensation, Claisen condensation, Dieckmann • John E. McMurry, Organic Chemistry, Brooks Cole, 6th Edition, 2003 • John E. McMurry, Eric E. Simanek, Fundamentals of Organic Chemistry³, Brooks Cole, 6th Edition, 2006 Olefine/Olefin Another name for an alkene. PCC Pyridinium chlorochromate is a reagent developed by E. J. Corey which enables the conversion of primary alcohols to aldehydes. • Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers, Organic Chemistry, Oxford University Press, 2000 PDC Pyridinium dichromate, also known as the Cornforth reagent, is chemical similar to PCC in properties and utility, while being less acidic and thus more useful in some scenarios. • Norman L. Allinger et al, Organic Chemistry, Worth Publishers, 1973 Reagent A chemical used to effect a certain transformation • Stanley H. Pine, Organic Chemistry, McGraw-Hill Companies, 1987 Ylide A ylid or ylide (US) is a neutral molecule with a positive and a negative charge on adjacent atoms. ey appear in organic chemistry as reagents or reactive intermediates. • Francis A. Carey, Richard J. Sundberg, Advanced Organic Chemistry, Springer, 5th Edition, 2008 ReferencesforFurtherReading • K. Peter C. Vollhardt, Neil E. Schore, Organic Chemistry: Structure and Function, W. H. Freeman; 5th Edition, 2005 • T. W. Graham Solomons, Craig B. Fryhle, Organic Chemistry, Wiley, 9th Edition, 2007 • Stuart Warren, Organic Synthesis: e Disconnection Approach, Wiley, 1984 ³is McMurry book is much briefer, totalling at 640 pages. 8