QUALITATIVE TESTS FOR IDENTIFICATION OF CARBON
advertisement

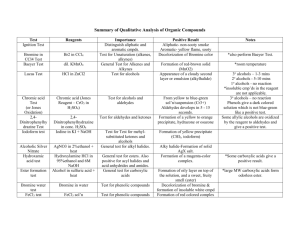
QUALITATIVE TESTS FOR IDENTIFICATION OF CARBON-CARBON UNSATURATION AND ALCOHOL FUNCTIONS (Rev’d 12/12/2004) In this experiment we will examine two reagents (bromine in carbon tetrachloride and aqueous potassium permanganate) which are widely used for qualitative identification of carbon-carbon unsaturation and three reagents (chromic acid, hydrochloric acid, and hydrochloric-zinc chloride) which are widely used for qualitative identification of alcohols. Please handle these reagents with care and avoid contact with skin. Bromine causes chemical burns, permanganate causes stubborn to remove stains, and chromic acid and Lucas reagent are very strongly acidic solutions. Reagents for Detection of Unsaturation Bromine in Carbon Tetrachloride This reagent is commonly used to identify the presence of olefinic or acetylenic functions. The red bromine color of this reagent is discharged when the unsaturated function reacts with the bromine. This reagent should be used in conjunction with the aqueous permanganate reagent to avoid erroneous conclusions. Erroneous results with the bromine reagent may occur for two reasons. Firstly, not all olefinic or acetylenic compounds take up bromine. When electron withdrawing groups are attached to the unsaturated carbon atoms the uptake of bromine is considerably slowed and in extreme cases completely stopped. C 6H5CH CH2 + C 6H5CH CHCO 2H fast Br2 + Br2 slow C 6H5CHBrCH 2Br C 6H5CHBrCHBrCO 2H Secondly, some other functionalities discharge the bromine color but with the evolution of hydrogen bromide. That is to say, they undergo bromine substitution instead of bromine addition. Compounds which undergo substitution extremely rapidly are phenols, enols, and anilines. Thus a positive test for unsaturation is one that discharges the bromine color without the evolution of hydrogen bromide. Procedure: Add 1 drop of the liquid (or the tip of a microspatula of a solid plus 3 drops of ethanol) to a small test tube and then add a 5% (0.3 M) solution of bromine in carbon tetrachloride drop by drop (with agitation) until the bromine color remains. Run this test on (1) cyclohexene, (2) cyclohexane, (3) ethanol, and (4) cinnamic acid and record your observations. For the cinnamic acid, let the test tube sit and check it periodically during the remainder of the lab period to see how long it takes for the bromine color to disappear. Aqueous Potassium Permanganate The purple permanganate color of this reagent is discharged by olefinic and acetylenic functions. This is known as the Baeyer test for unsaturation. In cold (room temperature or below) dilute aqueous solutions the main products from the reaction of an olefin with permanganate are the corresponding vicinal diol and manganese dioxide (brown solid). Acetylenes produce the corresponding diones. If heated these initial products undergo further oxidation, leading eventually to carbon chain cleavage with the formation of carboxylic acids. This test is very general and gives positive tests with those unsaturated compounds that do not decolorize bromine in carbon tetrachloride. RCH CHR + KMnO4 + 2 H2O RCH CHR + MnO2 + 2 KOH OH OH RCH CHR OH OH further oxidation 2 RCO 2H Two cautions are in order when using the Baeyer test. Firstly, aldehydes give positive tests because they are readily oxidized to carboxylic acids by permanganate. Secondly, although pure alcohols do not react within 5 minutes, they often contain trace impurities that are easily oxidized and give a slight reaction. Likewise alkanes often contain trace amounts of olefins as impurities and may give a slight reaction. For these reasons decolorization of a drop or two of the reagent should not be accepted as a positive test. Procedure: Add 4 drops of the liquid (or the tip of a microspatula of a solid) to be tested to 1 mL (~20 drops) of ethanol in a small test tube. Then add a 1% potassium permanganate solution drop by drop with shaking until a dark precipitate forms or the purple color of the permanganate persists. Run this test on the same compounds used in the bromine test above and record your observations. If decolorization doesn't occur in ~1 minute, allow the mixture to stand for 5 minutes with occasional shaking. Perform both the above tests with the two unknowns and report your observations and conclusions. Reagents for Detection and Classification of Alcohols Chromic Acid This reagent provides a rapid method for the detection of alcohol functions and also allows for distinguishing primary and secondary alcohols from tertiary alcohols. When a solution of the alcohol in acetone is treated with several drops of the chromic acid solution, the orange-red color of the chromic acid is disappeared and a green to blue opaque suspension of chromium(III) sulfate is formed. Primary and secondary alcohols react within 2 seconds but tertiary alcohols do not. Tertiary alcohols will eventually react when allowed to stand for longer periods of time in the strongly acidic solution. Aldehydes give a positive test, but olefins, acetylenes, amines, ethers, and ketones give negative tests within 2 seconds. RCH 2OH + H2CrO4 + H2SO 4 RCO 2H + Cr2(SO 4) 3 + H2O R2CHOH + H2CrO4 + H2SO 4 R2CO + Cr2(SO 4) 3 + H2O Procedure: Add 1 drop of the liquid to be tested to 1 mL of reagent grade acetone in a small test tube. Then add 1 drop of the chromic acid reagent and note the result within 2 seconds. A positive test is for the orange color of the chromic acid to be replaced by a green to blue opaque suspension. Run this test on (1) 1-butanol, (2) 2-butanol, and (3) 1,1-dimethylethanol (t-butanol) and record your observations. Hydrochloric Acid-Zinc Chloride (Lucas Reagent) Only tertiary alcohols and alcohols that can form carbocations of equal stability to tertiary carbocations react with concentrated hydrochloric acid to produce alkyl chlorides. However, alcohols are more reactive towards Lucas reagent than with concentrated hydrochloric acid. The zinc chloride is a potent Lewis acid and forms such a strong Lewis acid-base complex with the hydroxyl group that it becomes a better leaving group than even water. Thus Lucas reagent allows for distinguishing primary, secondary, and tertiary alcohols from one another. Primary alcohols are essentially inert towards Lucas reagent at ordinary temperatures, but secondary and tertiary alcohols are converted into their alkyl chlorides at easily discernible different rates by Lucas reagent. Because a positive test depends upon the appearance of the alkyl chloride as a second liquid phase, the test is clearly limited to alcohols that are soluble in the reagent. This usually limits the test to monofunctional alcohols with less than 6 carbons and to certain polyfunctional alcohols such as glycols. Tertiary alcohols give positive tests in less than 1 minute, whereas secondary alcohols begin to show milky emulsions within 5 minutes with a distinct layer visible in about 10 minutes. R 3COH HCl H R 3C O H H2O R3C + Cl R3CCl Cl R 2CHOH R 3COH ZnCl 2 ZnCl 2 H Cl R 2CH H R 3C O ZnCl O ZnCl HOZnCl HOZnCl R 3C + Cl R 2CHCl R3CCl Cl Procedure: To 3 drops of the alcohol in a small test tube add 1 mL of Lucas reagent which has been warmed to ~25 oC. Using a gloved finger, stopper the tube and shake. Allow the mixture to stand and note the time required for the formation of the alkyl chloride, which appears as a milky emulsion or a second layer. Run this test on (1) 1-butanol, (2) 2butanol, (3) 1,1-dimethylethanol (t-butanol) and record your observations. Repeat this procedure using concentrated hydrochloric acid in place of the Lucas reagent and record your observations. Perform the tests for detection and classification of alcohols with the two unknowns given by your TA and report your observations and conclusions. Record the unknown code in your lab notebook. Lab Notebook Preparation Prior to lab, prepare tables similar to the example provided for each of the five tests that are to be performed during the lab period. These will serve for all of your data and observation entries. Conclusions should also be entered directly into the tables and turned in by the end of the lab period you complete the experiment in. Some points, which are normally assigned to the postlab exercise, have been redistributed to these conclusions. The experiment’s Postlab Exercise is due one week following the completion of the experiment. NOTE: Some of the reactions can (or should be) observed immediately; others should be observed over a period of time. WASTE DISPOSAL INFORMATION 1. Detection of Unsaturation Tests a. Br2 in CCl4 TEST: Discard ALL test solutions in Halogenated Waste Container. b. KMnO4 (aq) TEST: Discard ALL test solutions in NON-halogenated Waste Container. 2. DETECTION & CLASSIFICATION OF ALCOHOLS Use sink for disposal (with plenty of running water). Name:_______________________________Section:________ Date:___________ POSTLAB EXERCISES FOR: QUALITATIVE TESTS FOR IDENTIFICATION OF CARBONCARBON UNSATURATION AND ALCOHOL FUNCTIONS (Rev’d 12/12/2004) Due in lab one week following the scheduled experiment. Please answer questions on this form. SUMMARY (21pts) The summary is in the form of conclusions for each of the tests performed and (should be) included in your notebook. 1. Conclusions for the unsaturation tests (3pts/test = 12pts). 2. Conclusions for the alcohol tests (3pts/test = 9pts). QUESTIONS (9pts) Based upon the background information provided on the above qualitative tests and your observations answer the following two questions on this form. 1. (3pts) A compound that is known to be one of the three shown below gives a negative bromine test, but a positive permanganate test. Which is the correct structure? OH CH3CH2CH2C(CH 3) 2 A CH3CH2 H C HO2C C C CH2CH3 H B H C CO2H H C 2. (6pts) An alcohol gives a positive Lucas test, but does not react with concentrated hydrochloric acid. Would you expect this alcohol to give a positive or negative chromic acid test? Explain.