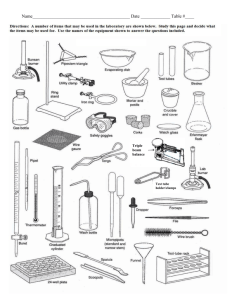
Expt EX 1 Exothermic Reaction (EX) Objective The purpose of this
advertisement

Expt EX 1 Exothermic Reaction (EX) Objective The purpose of this experiment is to determine the enthalpy change ∆H (kJ/mol) for the reaction NaClO(aq) + Na2SO3(aq) => NaCl(aq) + Na2SO4(aq) + heat The exothermic reaction of sodium hypochlorite (bleach) with sodium sulfite produces heat. Heat and heat capacity In this experiment, you will use a digital temperature probe to monitor the temperature changes, ∆T, during the course of (Part I) the chemical reaction reaction and (Part II) resistance heating. In Part II you will increase the temperature of the system using an electrical heater to determine the constant pressure heat capacity, Cp, of the solution. Determining qheating and ∆Theating gives Cp from Cp = ( qheating / ∆Theating ) (1) In Part I you will use the Cp determined from Part II and the measured ∆Treaction to determine ∆H from value for heat released by chemical reaction (qreaction) where qreaction = – Cp ∆Treaction (2) The negative sign is necessary because if heat is absorbed by solution then ∆Treaction is positive and then the qreaction must be exothermic and negative. The heat released by reaction (q is -) is the same heat absorbed by solution (q is +). Caution and Safety Keep all chemicals away from electronic devices. Undiluted bleach is irritating to the skin and harmful to eyes. Wash your hands after working with the NaClO solutions. Expt EX Procedure - Part I 2 Chemical Reaction Prepare 500mL of 0.400M sodium sulfite (Na2SO3) solution. Use a magnetic stirrer to help dissolve quickly and completely. Get a 50ml pipet ready so you can add sodium sulfite solution to the reaction beaker. Prepare 500mL of 0.300M sodium hypochlorite solution by diluting Chlorox bleach with water. Chlorox bleach is 6.00% by weight NaClO, and it has a density of 1.07 g/mL. Get a 100ml graduated cylinder ready to add NaClO solution to the reaction beaker. Do dilution calculations before you come to lab. You are going to add the two reactants to the reaction beaker and use the digital temperature probe to monitor the temperature as a function of time. Using the graduated cylinder, place 100mL of the NaClO solution into the reaction beaker (a 250mL beaker covered with cotton and tape for insulation). Place a stir bar in the bottom of the beaker, and position the large cork or rubber stopper onto the beaker so that the resistor heater and temperature probe are in position. Stir the solution slowly with the magnetic stirrer, making certain that the probe does not touch the bar or the resistor heater. Wait about a minute to allow time for solution temperature to equilibrate and then begin taking temperature readings (set at about 5second intervals). You need at least 60 seconds of temperature readings to make sure that the initial temperature has equilibrated to a constant value and is not changing. While the temperature readings continue to be automatically collected, you will add solution observe temperature change. Using the pipet, add exactly 50mL of the sodium sulfite solution through hole in middle of the stopper. You should see the temperature increase. Continue taking temperature readings for at least 60 seconds after the maximum temperature is reached. Observe your graph of temperature versus time, and determine the initial temperature and final (highest) temperature of the reaction system. Show the lab instructor this graph on the computer screen for the first reaction so it can be checked prior to continuing. Expt EX 3 Determine the mass of the liquid in the beaker because you will need this value in Part II. Discard the liquid in the beaker and rinse. Do the reaction a second time using the two solutions as you had done previously. Do the reaction a third time. Procedure – Part II Electric Heating Use a multimeter to determine the resistance of the electric heater that goes into the reaction beaker. Just set the multimeter to "resistance", turn on the multimeter, and connect the multimeter leads to the heater wires (it doesn't matter which lead goes on which wire). Record the resistance in Ohms, turn off the multimeter, and disconnect the leads from the heater. Place a mass of distilled water equal to mass of liquid used in Part I into the empty reaction beaker, and turn on the magnetic stirrer. Position the cork stopper so that the electric heater and temperature probe are immersed in the water. You will burn up the heater if you pass current through it when it isn't under water. With the DC power supply turned off, connect the two leads from the DC power supply to the electric heater. Begin taking temperature readings and continue until for at least 60 seconds to obtain value for the initial temperature. Remember to stir at same rate as in all prior reactions. Turn on the DC power supply and set the potential to 10.0 Volts by adjusting both the voltage and current settings. Pass current through the electric heater for exactly 5 minutes (measure exact number of seconds power is on). You should observe the temperature slowly increase. Turn off the DC power supply, and allow the temperature probe to continue taking measurements for at least 60 more seconds or until you have a clear maximum value for the final temperature. Repeat this electrical heating a second time with the same water and set up. Deionized water is used instead of the actual solution to avoid any electroysis reactions if the resistance heater has a bare wire. You can check your results based on Eqs. (3) and (4) to make sure you are getting a value close to the heat capacity expected for mass of water used which is = (water mass g) x ( 4.184J/gK) Expt EX 4 Calculations The heat q produced by the electrical heating is given by qheating = (V2)(t)/(R) (3) where V is the potential (volts), t is the time of heating in seconds, and R is the resistance (ohms). Recall that J = kg m2 s-2, V= kg m2 s-2 C-1, Ω= kg m2 s-1 C-2 where J is Joule, V is volt, Ω is ohm, and C is coulomb. Using the SI units above determine the units for , qheating in Eq (3). The change in temperature caused by the electric heating is given by qheating = Cp (T4 - T3) (4a) or solving for heat capacity Cp = qheating / (T4 - T3) (4b) where Cp is the heat capacity of the system (composed primarily of the solution but also with small possible contributions of heat adsorbed by the glass container, probe, resistance heater). T4 is the temperature after heating and T3 is the temperature before heating. Hence, knowing qheating from Eq (3) and T4 and T3 you can calculate the heat capacity Cp of the system (reaction beaker plus contents) using Eq (4). The heat capacity Cp of the system stays the same as long as the volume of liquid in the reaction beaker is constant. Hence, the value of the heat capacity Cp calculated from data in Part II also applies to Part I. Expt EX 5 Therefore, the heat produced by the exothermic reaction in Part I is given by qreaction = – Cp (T2 -T1) (5) where Cp is the heat capacity of the system determined in Part II, T2 is the temperature after reaction, and T1 is the temperature before reaction. The negative sign is present in the equation because the heat adsorbed by the solution is positive because the temperature increases from T1 to T2 then the heat of the chemical reaction must be negative. A negative value of q indicates an exothermic reaction. The Heat of Reaction, ∆H (kJ/mol) can be calculated from the relation ∆H = qreaction / n (4) where, n is the moles of the limiting reactant and for exothermic reactions, qreaction (J) and ∆H (kJ/mol) are both negative values. To find n you will need to determine the moles of NaClO(aq) and Na2SO3(aq) and find which is the limiting reactant. Make appropriate tables of all your data showing all the measured values Ti and Tf and ∆T. You do not need to include any T versus t graphs. When multiple determinations are made then average results to present final best value. Refer to thermodynamic tables to find literature values for heats of formation of aqueous salts and ions and use these to determine the expected heat of reaction ∆Hreaction (kJ/mol). You should be aware that thermodynamic tables may be expressed in kcal/mol or kJ/mol so you must check and determine the units used in your table. Do not assume the units are what you want. Show these values and calculation in your report. Compare this to your experimental value. Don't guess about the units in values found in tables - know them. Also in thermodynamic tables Expt EX 6 you must select the same chemical in the same physical state. For example, in considering enthalpies of formation (∆Hf), ∆Hf for NaCl(s) is not the same as ∆Hf for NaCl(aq). Return the lab equipment and supplies obtained from the lab drawer to that drawer. Leave equipment originally set up on bench as it was for next group of lab students. Make sure that you have the initial temperature and final temperature from all your graphs and then you will not need to print data table or graphs. You do not need all the data point values for your analysis.