Learning Objectives General Chemistry II (CHEM 1474) Buckley
advertisement

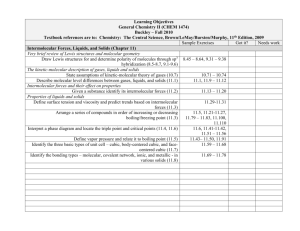
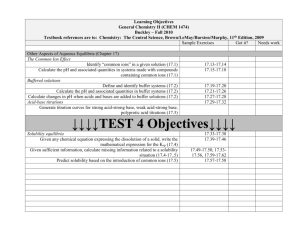
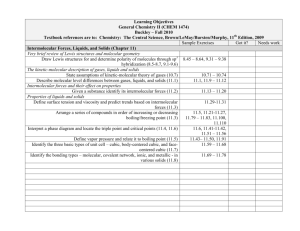
Learning Objectives General Chemistry II (CHEM 1474) Buckley – Fall 2010 Textbook references are to: Chemistry: The Central Science, Brown/LeMay/Bursten/Murphy, 11th Edition, 2009 Sample Exercises Got it? Needs work Intermolecular Forces, Liquids, and Solids (Chapter 11) Very brief review of Lewis structures and molecular geometry Draw Lewis structures for and determine polarity of molecules through sp3 8.45 – 8.64, 9.31 – 9.38 hybridization (8.5-8.7, 9.1-9.6) The kinetic-molecular description of gases, liquids and solids State assumptions of kinetic-molecular theory of gases (10.7) 10.71 – 10.74 Describe molecular level differences between gases, liquids, and solids (11.1) 11.1, 11.9 – 11.12 Intermolecular forces and their effect on properties Given a substance identify its intermolecular forces (11.2) 11.13 – 11.20 Properties of liquids and solids Define surface tension and viscosity and predict trends based on intermolecular 11.29-11.31 forces (11.3) Arrange a series of compounds in order of increasing or decreasing 11.5, 11.21-11.27, 11.79 – 11.83, 11.100, boiling/freezing point (11.3) 11.110 Interpret a phase diagram and locate the triple point and critical points (11.4, 11.6) 11.6, 11.41-11.42, 11.51 – 11.56 Define vapor pressure and relate it to boiling point (11.5) 11.43– 11.50, 11.91 Identify the three basic types of unit cell – cubic, body-centered cubic, and face11.59 – 11.68 centered cubic (11.7) Identify the bonding types – molecular, covalent network, ionic, and metallic - in 11.69 – 11.78 various solids (11.8) Learning Objectives General Chemistry II (CHEM 1474) Buckley – Fall 2010 Textbook references are to: Chemistry: The Central Science, Brown/LeMay/Bursten/Murphy, 11th Edition, 2009 Sample Exercises Got it? Needs work Properties of Solutions (Chapter 13) The solution process - energetics Identify the endothermicity and exothermicity of steps in the solution process 13.13-13.14 (13.1) Identify the role of entropy in the solution process (13.1) 13.3 Define the terms unsaturated, saturated, and supersaturated (13.2) 13.5, 13.21-13.26 Predict solubilities based on intermolecular forces (13.3) 13.7, 13.15-13.16, 13.29-13.30, 13.87 State and predict effects of temperature and pressure on 13.8, 13.31-13.33, solubility (13.3) 13.89-13.90 Methods of expressing concentrations Work numerically with the expressions of mass percentage, ppm, ppb, molarity, 13.9, 13.35-13.56 molality, and mole fraction (4.5, 13.4) Colligative properties Describe the four colligative properties of solutions and write the mathematical relationships that apply to each (13.5) Apply Raoult’s Law and the relationships between concentration and freezing 13.10-13.11, 13.57point, boiling point, and osmosis to a variety of problem situations (13.5) 13.80, 13.96, 13.10013.103 Colloids Qualitatively describe colloids (13.6) 13.83-13.86 Learning Objectives General Chemistry II (CHEM 1474) Buckley – Fall 2010 Textbook references are to: Chemistry: The Central Science, Brown/LeMay/Bursten/Murphy, 11th Edition, 2009 Sample Exercises Got it? Needs work Chemical Kinetics (Chapter 14) Methods of expressing rates Given a chemical reaction, express the rate in terms of the stoichiometry (14.2) 14.1-14.4 Factors that affect reaction rates Identify factors that affect a reaction rate and their effect (14.1) Given experimental initial rate information, determine the rate law for a reaction 14.13-14.34 (14.3) Given sufficient concentration/time information, find missing information for 14.35-14.46 first- and second- order reactions (14.4) Use half-life relationships for first- and second-order reactions to find missing 14.6 information (14.4) Describe molecularly the effect of temperature on reaction rate (14.5) 14.5,14.7 Apply the Arrhenius equation (14.5) 14.47-14.60 Qualitatively describe the mechanisms of homogeneous, heterogeneous, and enzyme catalysis (14.7) Mechanisms and molecularity Identify the molecularity of an elementary step 14.61-14.64 Combine given chemical equations to result in a desired chemical equation 14.67, 14.70 Learning Objectives General Chemistry II (CHEM 1474) Buckley – Fall 2010 Textbook references are to: Chemistry: The Central Science, Brown/LeMay/Bursten/Murphy, 11th Edition, 2009 Sample Exercises Got it? Needs work Chemical Equilibrium (Chapter 15) The concept of equilibrium Describe the concept of a dynamic equilibrium (15.1) The equilibrium constant Write Kc and Kp for a given system to include heterogeneous systems (15.3-15.4) 15.11-15.14 Relate Kc and Kp (15.2) 15.17-15.18 Interpreting and working with equilibrium constants Relate chemical equations and equilibrium constants (reverse, take reciprocal, 15.19-15.26 etc.) (15.3) Given sufficient information, determine whether or not a system is at equilibrium 15.2-15.7 (15.2) Predict direction of equilibrium (to the left, to the right) based on value of 15.15-15.16 equilibrium constant (15.3) Calculating equilibrium constants Find the value of the equilibrium constant given equilibrium concentrations or 15.27-15.34 missing equilibrium concentrations given sufficient information (15.5) Use the equilibrium constant and given concentrations to predict the direction of 15.35 – 15.38 reaction (reaction quotient) (15.6) Calculate equilibrium concentrations given starting concentrations and the 15.39-15.50 equilibrium constant (15.6) LeChâtelier's Principle Predict shifts in equilibrium due to changes in concentration, volume, temperature, 15.8-15.9, 15.51-15.56 pressure, or the addition of catalyst (15.7) (in 15.50, CaCrO4 should be a solid) Learning Objectives General Chemistry II (CHEM 1474) Buckley – Fall 2010 Textbook references are to: Chemistry: The Central Science, Brown/LeMay/Bursten/Murphy, 11th Edition, 2009 Sample Exercises Got it? Needs work Acid-base equilibria (Chapter 16) Review of current acid-base concepts Arrhenius Theory (16.1) 16.15-16.16 Identify substances as Bronsted-Lowry acids or bases (16.2) Identify conjugate acid-base pairs (16.2) 16.17-16.24 Determine relative acidity or basicity of substances (16.2) 16.25-16.28, 16.107 The autoionization of water Given hydronium or hydroxide concentration, determine the other (16.3) 16.29-16.34 The pH scale Given hydronium, hydroxide, pH, and/or pOH determine the other values (16.4) 16.35-16.42, 16.111 Calculations involving strong acids and bases Determine hydronium, hydroxide, pH, and/or pOH values given a concentration of 16.43-16.50, 16.110, a strong acid or base (16.5) 16.112, 16.122-16.123 Calculations involving weak acids Given sufficient information, determine hydronium, hydroxide, pH, pOH, Ka, 16.51-16.70, 16.105and/or percent ionization for a weak acid solution (16.6) 16.106, 16.113-16.115, 16.130 Calculations involving weak bases Given sufficient information, determine hydronium, hydroxide, pH, pOH, Ka, 16.71-16.78, 16.118and/or percent ionization for a weak base solution (16.7) 16.119,16.127 Relationship between Ka and Kb Determine Ka and Kb for a conjugate acid/base pair given one of them (16.8) 16.79-16.82 Acid-base properties of salt solutions Given sufficient information, determine hydronium, hydroxide, pH, pOH, Ka, 16.83-16.90, 16.116 and/or percent ionization for a salt solution (16.9) Acid-base behavior and chemical structure Identify factors that affect acid and base strengths (16.10) 16.91-16.92 Evaluate the relative acidity or basicity of a series of compounds (16.10) 16.93-16.98, 16.120 Lewis acids and bases Identify materials as Lewis acids or bases (16.11) 16.99-16.104, 16.125