Exp2-Recrystallization and Melting Point Procedure
advertisement
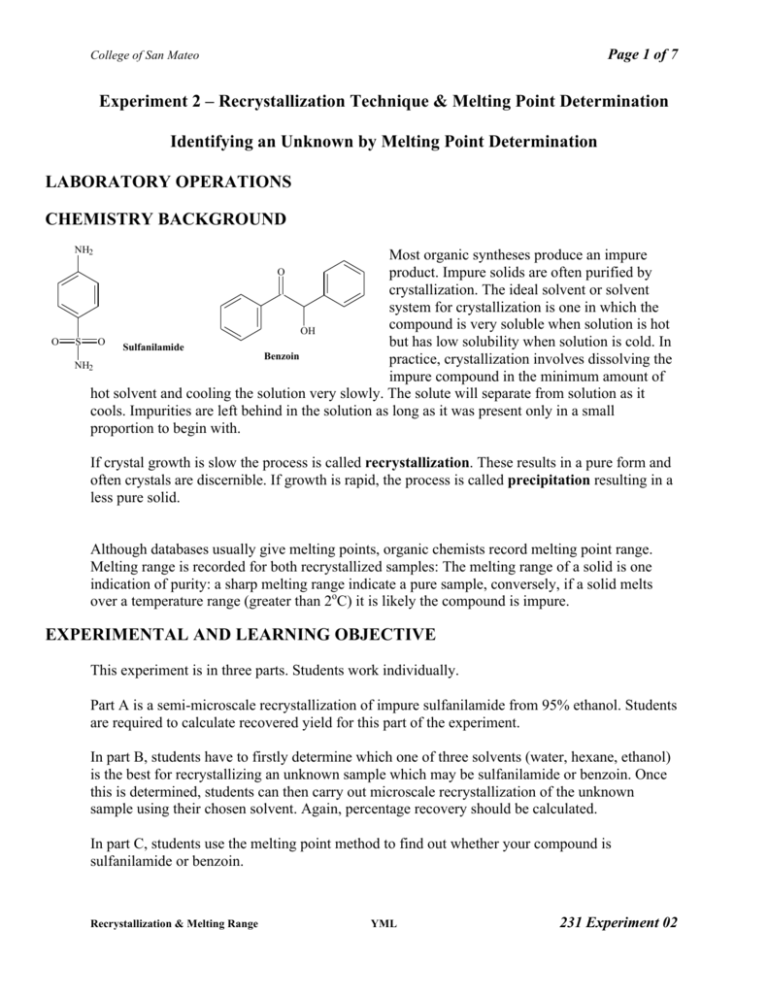
Page 1 of 7 College of San Mateo Experiment 2 – Recrystallization Technique & Melting Point Determination Identifying an Unknown by Melting Point Determination LABORATORY OPERATIONS CHEMISTRY BACKGROUND NH2 Most organic syntheses produce an impure product. Impure solids are often purified by crystallization. The ideal solvent or solvent system for crystallization is one in which the compound is very soluble when solution is hot OH S O but has low solubility when solution is cold. In Sulfanilamide Benzoin practice, crystallization involves dissolving the NH2 impure compound in the minimum amount of hot solvent and cooling the solution very slowly. The solute will separate from solution as it cools. Impurities are left behind in the solution as long as it was present only in a small proportion to begin with. O O If crystal growth is slow the process is called recrystallization. These results in a pure form and often crystals are discernible. If growth is rapid, the process is called precipitation resulting in a less pure solid. Although databases usually give melting points, organic chemists record melting point range. Melting range is recorded for both recrystallized samples: The melting range of a solid is one indication of purity: a sharp melting range indicate a pure sample, conversely, if a solid melts over a temperature range (greater than 2oC) it is likely the compound is impure. EXPERIMENTAL AND LEARNING OBJECTIVE This experiment is in three parts. Students work individually. Part A is a semi-microscale recrystallization of impure sulfanilamide from 95% ethanol. Students are required to calculate recovered yield for this part of the experiment. In part B, students have to firstly determine which one of three solvents (water, hexane, ethanol) is the best for recrystallizing an unknown sample which may be sulfanilamide or benzoin. Once this is determined, students can then carry out microscale recrystallization of the unknown sample using their chosen solvent. Again, percentage recovery should be calculated. In part C, students use the melting point method to find out whether your compound is sulfanilamide or benzoin. Recrystallization & Melting Range YML 231 Experiment 02 Page 2 of 7 College of San Mateo DIRECTIONS • • • Instructor will demonstrate operations as necessary. Read procedure sections from lab textbook as listed above. Answer prelab questions – see Attached sheet. Please answer in short phrases, no need to give essay answers. SAFETY: Sulfanilamide is an irritant, wear gloves and goggles. Assume all unknown compounds to be toxic, handle with care. PROCEDURE Part A –Recrystallization of sulfanilamide 1) Weigh 0.3g of impure sulfanilamide and transfer this solid to a 10 mL Erlenmeyer flask. Record exact amount of solid used. 2) Measure out approx. 20 mL of 95% ethanol into a 25mL Erlenmeyer flask – this is your supply of ethanol for part A. 3) Gently heat the 95% ethanol until it is hot. 4) Add a small amount of the hot 95% ethanol to the flask containing the sulfanilamide. (Approx 4 to 5 mL, using a calibrated Pasteur pipet). 5) Gently heat the flask slowly on a warm hot plate until boiling. Swirl occasionally to avoid bumping. At the same time, keep the remaining 10-15mL 95% ethanol warm on the same hotplate. Meanwhile, chill about 2-3 mL of 95% ethanol in a test tube ready for washing during vacuum filtration. 6) As the solution almost boils, the solid should dissolve. If there is excessive loss of solvent due to evaporation, add more solvent. If solids remain even when solution is boiling gently, add more solvent. When all solid is dissolved, remove solution from hot plate and allow it to cool slowly. 7) Crystallization should have begun as the solution cools. If no crystals appear after a few minutes, scratch the inside surface of the solution with a glass rod to induce crystallization. Alternatively, crystallization may be induced by “seeding”. Recrystallization & Melting Range YML 231 Experiment 02 Page 3 of 7 College of San Mateo When the amount of crystals has stopped increasing at room temperature, place flask in an icebath using a beaker for further crystallization. 8) Collect crystals by vacuum filtration using a Hirsch funnel. Moisten filter paper with a few drops of chilled 95% ethanol. Turn on vacuum. Swirl mixture in the flask and quickly pour into the funnel. 9) Add about 1 mL of chilled ethanol to the flask, swirl and once again pour into funnel. This will wash out any remaining solid from the flask and also wash the already collected crystals too. If necessary, repeat washing step. 10) Continue to draw air through the Hirsch funnel for 30-60 sec to further dry the crystals. Transfer to a preweighed small container for air-drying. If the crystals are in large clumps, break up with a spatula carefully to assist drying. Determine “wet” mass of sulfanilamide and label container with your name, date and name of solid. 11) Next lab session: reweigh container to obtain “dry” mass of recrystallized sulfanilamide and determine its melting point alongside a sample of impure sulfanilamide. Note any difference in how the two samples melt. Part B – Recrystallization of Unknown Solid 12) Place an aluminum block with a small opening for small tubes on a hotplate set on medium heat. 13) Place approx. 5 mL each of water, ethanol and hexane in small flasks and label flask. These are your supply of solvents for part B. (You may wish to double quantities and share with your fume hood partner). 14) Place a small amount (approx. 30-50mg – tip of small spatula) of impure unknown solid in each of three small test tubes. 15) To each test tube, add about 1ml of water, ethanol or hexane. Label each tube so you know which solvent is used in which test tube. 16) Gently shake all three test tubes to see if unknown solid dissolves in cold solvent. If it does, then that solvent is not suitable and you may discard it either now or later. 17) For any test tubes which still contains undissolved solid, place into aluminum block. Again, gently shake test tubes as the solvents heat up. When solvents warm up, check to see which solvent has completely dissolved its solid. 18) If necessary, add a few more drops of the correct solvent if necessary. Then wait until the solution almost boils (but don’t let it boil) again before deciding whether all solid has dissolved. Recrystallization & Melting Range YML 231 Experiment 02 Page 4 of 7 College of San Mateo (If in any test tube not all the solid has dissolved by the time 2-3 mL of solvents has been added, then this solvent is also not appropriate for recrystallization.) 19) Once all solid has dissolved, remove test tube from hotplate and allow to cool to room temperature. 20) Crystallization should have begun as the solution cools. If no crystals appear after a few minutes, scratch the inside surface of the solution with a glass rod to induce crystallization. Alternatively, crystallization may be induced by “seeding”. 21) If crystallization does occur (either assisted or not), then this solvent is suitable for recrystallizing the unknown. 22) Weigh 0.3g of impure unknown compound and transfer this solid to a 25 mL Erlenmeyer flask. Record exact amount of solid used. 23) Measure out approx. 20 mL of your chosen solvent into a 25mL Erlenmeyer flask – this is your supply of solvent. 24) Add a boiling chip to the solvent and gently heat the solvent until it is hot. 25) Add a small amount of the hot solvent to the flask containing the unknown so that the solid is just covered by the solvent. (Approx 4 to 5 mL, using a calibrated Pasteur pipet). 26) Gently heat the flask slowly on a warm hot plate until boiling. Swirl occasionally to avoid bumping. At the same time, keep the remaining 10-15mL of solvent warm on the same hotplate. Meanwhile, chill about 2-3 mL of your chosen solvent in a test tube ready for washing during vacuum filtration. 27) As the solution almost boils, the solid should dissolve. If there is excessive loss of solvent due to evaporation, add more solvent. If solids remain even when solution is boiling gently, add more solvent. When all solid is dissolved, remove solution from hot plate and allow it to cool slowly. 28) Crystallization should have begun as the solution cools. If no crystals appear after a few minutes, scratch the inside surface of the solution with a glass rod to induce crystallization. Alternatively, crystallization may be induced by “seeding”. 29) Chill the test tube where crystals have begun to form. Also chill 3-5 mL of the recrystallization solvent as determined in an ice-bath ready for washing filtered crystals. 30) Set up for vacuum filtration. Filter recrystallized solids, use 2 portions of 1mL chilled solvent to rinse out any solid and wash solids in the filtration flask. Recrystallization & Melting Range YML 231 Experiment 02 Page 5 of 7 College of San Mateo 31) Let solid air dry for 2-3 minutes before transferring to a small, preweighed container. 32) Calculate mass of “wet” solid obtained and label container with your name, date and name of solid. 33) Next lab session: reweigh container to obtain “dry” mass and use crystals of unknown in part C. Procedure Part C – Determine whether unknown is sulfanilamide or Benzoin. 34) Take a small amount of your unknown solid and mix it thoroughly with an equal amount of sulfanilamide. If necessary, crush the crystals using a piece of folded weighing paper to ensure good mixing. Fill a melting point tube with this mixture. 35) Repeat step 34 with a small amount of unknown and benzoin. 36) Record melting point range of both mixtures and decide whether the unknown sample is sulfanilamide or benzoin. Disposal & Cleaning • • • Rinse all used glassware with acetone in your fumehood before washing in water. Transfer waste acetone wash to waste station. Wipe fumehood with damp paper towels Working Notes • • • • Your report should have your name and date the work was done, a title indicating scenario and/or experimental objective(s). Include any relevant data and information such as chemical structures or reaction equation. Report and interpret relevant evidence – including observations, data and measurements and calculations. Calculations of yields must be clearly laid out. State your conclusion, and explain how your evidence supports this. Recrystallization & Melting Range YML 231 Experiment 02 Page 6 of 7 College of San Mateo Name: ___________________________ Credit Points: _________ Prelab Questions: Recrystallization and Melting Range Prelab Questions 1. What is the purpose of recrystallizing a solid compound? 2. Describe the characteristics of a suitable solvent for recrystallization. (Hint: “Insoluble when cold……..”) 3. Suppose in part C, you used 0.955g of benzoin and recovered 0.653g of pure, recrystallized benzoin, calculate the % recovery. 4. In laboratory work, you usually obtain a melting range rather than a melting point. Explain what is a melting range. 5. With respect to the recrystallization process, why should you be careful to only add the minimal amount of solvent to the crude product? (Consider what could happen if you add too much solvent.) Recrystallization & Melting Range YML 231 Experiment 02 Page 7 of 7 College of San Mateo Name: ___________________________ Credit Points: _________ Postlab Questions: Recrystallization and Melting Range 1. Record the melting points for your recrystallized sulfanilamide from part A and compare the values to the literature values. Comment on the purity of the samples you recrystallized. 2. Report the % recovery of your products that you recrystallized. Comment on the yields and whether recrystallization is an efficient purification method. 3. Why is it important to let the crystals form slowly rather than immediately putting your solution into an ice bath? 4. Why is it important to use an Erlenmeyer flask rather than a beaker when dissolving the solid during a recrystallization? 5. Isolation of the crystals was done by vacuum filtration. Why is it important to use chilled solvent to wash the isolated crystals? (Consider what would happen if you used room temperature or warm solvent to rinse the crystals) Recrystallization & Melting Range YML 231 Experiment 02






